Atomic-Level View of the Functional Transition in Vertebrate Hemoglobins: The Case of Antarctic Fish Hbs
- PMID: 35930673
- PMCID: PMC9400108
- DOI: 10.1021/acs.jcim.2c00727
Atomic-Level View of the Functional Transition in Vertebrate Hemoglobins: The Case of Antarctic Fish Hbs
Abstract
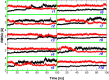
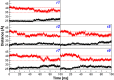
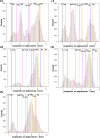
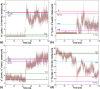
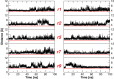
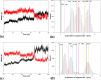
Tetrameric hemoglobins (Hbs) are prototypal systems for studies aimed at unveiling basic structure-function relationships as well as investigating the molecular/structural basis of adaptation of living organisms to extreme conditions. However, a chronological analysis of decade-long studies conducted on Hbs is illuminating on the difficulties associated with the attempts of gaining functional insights from static structures. Here, we applied molecular dynamics (MD) simulations to explore the functional transition from the T to the R state of the hemoglobin of the Antarctic fish Trematomus bernacchii (HbTb). Our study clearly demonstrates the ability of the MD technique to accurately describe the transition of HbTb from the T to R-like states, as shown by a number of global and local structural indicators. A comparative analysis of the structural states that HbTb assumes in the simulations with those detected in previous MD analyses conducted on HbA (human Hb) highlights interesting analogies (similarity of the transition pathway) and differences (distinct population of intermediate states). In particular, the ability of HbTb to significantly populate intermediate states along the functional pathway explains the observed propensity of this protein to assume these structures in the crystalline state. It also explains some functional data reported on the protein that indicate the occurrence of other functional states in addition to the canonical R and T ones. These findings are in line with the emerging idea that the classical two-state view underlying tetrameric Hb functionality is probably an oversimplification and that other structural states play important roles in these proteins. The ability of MD simulations to accurately describe the functional pathway in tetrameric Hbs suggests that this approach may be effectively applied to unravel the molecular and structural basis of Hbs exhibiting peculiar functional properties as a consequence of the environmental adaptation of the host organism.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
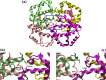
Similar articles
-
Combined crystallographic and spectroscopic analysis of Trematomus bernacchii hemoglobin highlights analogies and differences in the peculiar oxidation pathway of Antarctic fish hemoglobins.Biopolymers. 2009 Dec;91(12):1117-25. doi: 10.1002/bip.21206. Biopolymers. 2009. PMID: 19373928
-
Quaternary Structure Transitions of Human Hemoglobin: An Atomic-Level View of the Functional Intermediate States.J Chem Inf Model. 2021 Aug 23;61(8):3988-3999. doi: 10.1021/acs.jcim.1c00315. Epub 2021 Aug 10. J Chem Inf Model. 2021. PMID: 34375114 Free PMC article.
-
Protonation of histidine 55 affects the oxygen access to heme in the alpha chain of the hemoglobin from the Antarctic fish Trematomus bernacchii.IUBMB Life. 2011 Mar;63(3):175-82. doi: 10.1002/iub.436. IUBMB Life. 2011. PMID: 21445848
-
Spectroscopic and crystallographic characterization of bis-histidyl adducts in tetrameric hemoglobins.Methods Enzymol. 2008;436:425-44. doi: 10.1016/S0076-6879(08)36024-8. Methods Enzymol. 2008. PMID: 18237647 Review.
-
Occurrence and formation of endogenous histidine hexa-coordination in cold-adapted hemoglobins.IUBMB Life. 2011 May;63(5):295-303. doi: 10.1002/iub.446. Epub 2011 Apr 13. IUBMB Life. 2011. PMID: 21491555 Review.
References
-
- Senior A. W.; Evans R.; Jumper J.; Kirkpatrick J.; Sifre L.; Green T.; Qin C.; Žídek A.; Nelson A. W. R.; Bridgland A.; Penedones H.; Petersen S.; Simonyan K.; Crossan S.; Kohli P.; Jones D. T.; Silver D.; Kavukcuoglu K.; Hassabis D. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. 10.1038/s41586-019-1923-7. - DOI - PubMed
-
- Tunyasuvunakool K.; Adler J.; Wu Z.; Green T.; Zielinski M.; Žídek A.; Bridgland A.; Cowie A.; Meyer C.; Laydon A.; Velankar S.; Kleywegt G. J.; Bateman A.; Evans R.; Pritzel A.; Figurnov M.; Ronneberger O.; Bates R.; Kohl S. A. A.; Potapenko A.; Ballard A. J.; Romera-Paredes B.; Nikolov S.; Jain R.; Clancy E.; Reiman D.; Petersen; Senior A. W.; Kavukcuoglu K.; Birney E.; Kohli P.; Jumper J.; Hassabis D. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. 10.1038/s41586-021-03828-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

