Real-world effectiveness of caplacizumab vs the standard of care in immune thrombotic thrombocytopenic purpura
- PMID: 35930694
- PMCID: PMC9792393
- DOI: 10.1182/bloodadvances.2022008028
Real-world effectiveness of caplacizumab vs the standard of care in immune thrombotic thrombocytopenic purpura
Abstract
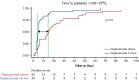
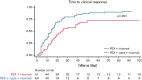
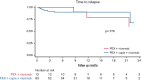
Immune thrombotic thrombocytopenic purpura (iTTP) is a thrombotic microangiopathy caused by anti-ADAMTS13 antibodies. Caplacizumab is approved for adults with an acute episode of iTTP in conjunction with plasma exchange (PEX) and immunosuppression. The objective of this study was to analyze and compare the safety and efficacy of caplacizumab vs the standard of care and assess the effect of the concomitant use of rituximab. A retrospective study from the Spanish TTP Registry of patients treated with caplacizumab vs those who did not receive it was conducted. A total of 155 patients with iTTP (77 caplacizumab, 78 no caplacizumab) were included. Patients initially treated with caplacizumab had fewer exacerbations (4.5% vs 20.5%; P < .05) and less refractoriness (4.5% vs 14.1%; P < .05) than those who were not treated. Time to clinical response was shorter when caplacizumab was used as initial treatment vs caplacizumab used after refractoriness or exacerbation. The multivariate analysis showed that its use in the first 3 days after PEX was associated with a lower number of PEX (odds ratio, 7.5; CI, 2.3-12.7; P < .05) and days of hospitalization (odds ratio, 11.2; CI, 5.6-16.9; P < .001) compared with standard therapy. There was no difference in time to clinical remission in patients treated with caplacizumab compared with the use of rituximab. No severe adverse event was described in the caplacizumab group. In summary, caplacizumab reduced exacerbations and refractoriness compared with standard of care regimens. When administered within the first 3 days after PEX, it also provided a faster clinical response, reducing hospitalization time and the need for PEX.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.E.M.-C. has received honoraria for consulting and as a speaker from Sobi, Amgen, Takeda, Sanofi, Novo Nordisk, Grifols, Novartis, CSL Behring, Werfen; and grant founding from Novo Nordisk, Amgen, Takeda, Alexion. C.P.I. has served as a consultant and provided expert testimony for Sanofi and Takeda. A.E.K.F. has received honoraria for consulting and as a speaker for Sanofi and Novartis. J.G.-A.P. has received honoraria for consulting and as a speaker for Sanofi. J.C. has received research funding from Cerus, Kawasumi Laboratories, and Sanofi; and has received speaker or advisory fees from Cerus, Fresenius Kabi, Grifols, MacoPharma, Sanofi, and TerumoBCT. R.G. has received honoraria from Sanofi for consulting activities. J.d.l.R. has served as a consultant and has provided expert testimony for Sanofi. J.d.R.-G. has received honoraria for consulting from Takeda and Sanofi. D.V. has received honoraria from Sanofi as speaker and for consulting activities. I.G.-S. has received honoraria for consulting and as a speaker from Terumo BCT, Takeda and Sanofi. J.R.R.M. has received honoraria for consultancy on advisory boards and as a speaker from Janssen, AbbVie, AstraZeneca, CSL-Behring, Takeda, and GSK. The remaining authors declare no competing financial interests.
Figures


References
-
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666. - PubMed
-
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–2846. - PubMed
-
- Pascual-Izquierdo C, Del Rio-Garma J, de la Rubia J, et al. Spanish Apheresis Group (GEA) and Spanish Thrombotic Thrombocytopenic Purpura Registry (REPTT) Incidence, diagnosis, and outcome of immune-mediated thrombotic thrombocytopenic purpura: a nationwide survey by the Spanish registry of thrombotic thrombocytopenic purpura. J Clin Apher. 2021;36(4):563–573. - PubMed

