Discovery of Orally Bioavailable and Brain-Penetrable Prodrugs of the Potent nSMase2 Inhibitor DPTIP
- PMID: 35930706
- PMCID: PMC9980655
- DOI: 10.1021/acs.jmedchem.2c00562
Discovery of Orally Bioavailable and Brain-Penetrable Prodrugs of the Potent nSMase2 Inhibitor DPTIP
Abstract
Extracellular vesicles (EVs) can carry pathological cargo and play an active role in disease progression. Neutral sphingomyelinase-2 (nSMase2) is a critical regulator of EV biogenesis, and its inhibition has shown protective effects in multiple disease states. 2,6-
Conflict of interest statement
The authors declare the following financial interests which may be considered as potential competing interests: A.P., N.H., B.S.S., and R.R. are listed as inventors in patent applications filed by Johns Hopkins Technology Ventures covering novel compositions of nSMase2 inhibitors and their utility.
Figures
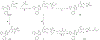
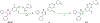
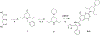
Similar articles
-
Pharmacokinetic Evaluation of Neutral Sphinghomyelinase2 (nSMase2) Inhibitor Prodrugs in Mice and Dogs.Pharmaceutics. 2024 Dec 26;17(1):20. doi: 10.3390/pharmaceutics17010020. Pharmaceutics. 2024. PMID: 39861669 Free PMC article.
-
Dendrimer-Conjugated nSMase2 Inhibitor Reduces Tau Propagation in Mice.Pharmaceutics. 2022 Sep 28;14(10):2066. doi: 10.3390/pharmaceutics14102066. Pharmaceutics. 2022. PMID: 36297501 Free PMC article.
-
DPTIP, a newly identified potent brain penetrant neutral sphingomyelinase 2 inhibitor, regulates astrocyte-peripheral immune communication following brain inflammation.Sci Rep. 2018 Dec 7;8(1):17715. doi: 10.1038/s41598-018-36144-2. Sci Rep. 2018. PMID: 30531925 Free PMC article.
-
Nipping disease in the bud: nSMase2 inhibitors as therapeutics in extracellular vesicle-mediated diseases.Drug Discov Today. 2021 Jul;26(7):1656-1668. doi: 10.1016/j.drudis.2021.03.025. Epub 2021 Mar 31. Drug Discov Today. 2021. PMID: 33798648 Free PMC article. Review.
-
[Advance in research on regulatory mechanism and functions of neutral sphingomyelinse 2].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013 Oct;35(5):581-5. doi: 10.3881/j.issn.1000-503X.2013.05.018. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013. PMID: 24183051 Review. Chinese.
Cited by
-
Discovery of tert-Butyl Ester Based 6-Diazo-5-oxo-l-norleucine Prodrugs for Enhanced Metabolic Stability and Tumor Delivery.J Med Chem. 2023 Nov 23;66(22):15493-15510. doi: 10.1021/acs.jmedchem.3c01681. Epub 2023 Nov 10. J Med Chem. 2023. PMID: 37949450 Free PMC article.
-
The Role of Neutral Sphingomyelinase-2 (NSM2) in the Control of Neutral Lipid Storage in T Cells.Int J Mol Sci. 2024 Mar 13;25(6):3247. doi: 10.3390/ijms25063247. Int J Mol Sci. 2024. PMID: 38542220 Free PMC article.
-
Blockade of neutral sphingomyelinase 2 exerts antitumor effect on metastatic castration resistant prostate cancer cells and promotes tumor regression when combined with Enzalutamide.Am J Cancer Res. 2024 Dec 15;14(12):5697-5716. doi: 10.62347/XXXA3182. eCollection 2024. Am J Cancer Res. 2024. PMID: 39803655 Free PMC article.
-
Melanoma-derived extracellular vesicles transfer proangiogenic factors.Oncol Res. 2025 Jan 16;33(2):245-262. doi: 10.32604/or.2024.055449. eCollection 2025. Oncol Res. 2025. PMID: 39866233 Free PMC article. Review.
-
Design, Synthesis, and Evaluation of Carbonate-Linked Halogenated Phenazine-Quinone Prodrugs with Improved Water-Solubility and Potent Antibacterial Profiles.ACS Infect Dis. 2023 Apr 14;9(4):899-915. doi: 10.1021/acsinfecdis.2c00558. Epub 2023 Mar 3. ACS Infect Dis. 2023. PMID: 36867688 Free PMC article.
References
-
- Luberto C; Hassler DF; Signorelli P; Okamoto Y; Sawai H; Boros E; Hazen-Martin DJ; Obeid LM; Hannun YA; Smith GK, Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem 2002, 277 (43), 41128–41139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

