Combined immunosuppression for acquired hemophilia A: CyDRi is a highly effective low-toxicity regimen
- PMID: 35930747
- PMCID: PMC10653022
- DOI: 10.1182/blood.2022016873
Combined immunosuppression for acquired hemophilia A: CyDRi is a highly effective low-toxicity regimen
Abstract
Acquired hemophilia A (AHA) is a rare severe autoimmune bleeding disorder with significant morbidity and mortality. Although critical for disease control, there is no consensus for the best immunosuppressive regimen. Most authors use steroids first line, followed by other agents for steroid failures. Upfront combined regimens offer the advantage of reduced steroid exposure and toxicity as well as increased efficacy. We retrospectively analyzed data from 32 patients with AHA treated on an identical such institutional protocol: cyclophosphamide 1000 mg on days 1 and 22, dexamethasone 40 mg on days 1, 8, 15, and 22, and rituximab 100 mg on days 1, 8, 15, and 22 (the regimen was termed CyDRi). All patients received at least 1 cycle of CyDRi. If necessary, CyDRi was repeated until remission, no sooner than day 43 of the previous cycle. Bleeding control was rapidly achieved. The median time for bleeding control was 15.5 days (range, 0-429 days; interquartile range, 2.5-29.5 days). Thirty-one (96.8%) of 32 patients achieved durable complete remission (CR); 29 (90.6%) of 32 patients were alive at last follow-up, all of them in CR. The median time to reach first CR was 77 days (range, 19-939 days; interquartile range, 31-115 days). Toxicity and side effects were acceptable and milder than those of commonly used, prolonged steroid therapies. In conclusion, the CyDRi regimen produced markedly higher CR rates and overall survival than currently used sequential regimens. Taken together, CyDRi proved to be an attractive option for the immunosuppression of elderly patients with AHA.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

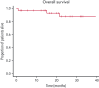


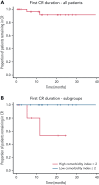
Comment in
-
Safer steps on a narrow path.Blood. 2022 Nov 3;140(18):1923-1924. doi: 10.1182/blood.2022017947. Blood. 2022. PMID: 36326794 No abstract available.
References
-
- Lozner EL, Jolliffe LS, Taylor FHL. About acquired hemophilia. Am J Med Sci. 1940;190:318.
-
- Collins PW, Hirsch S, Baglin TP, et al. UK Haemophilia Centre Doctors’ Organisation Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109(5):1870–1877. - PubMed
-
- Baudo F, de Cataldo F. Acquired hemophilia: a critical bleeding syndrome. Haematologica. 2004;89(1):96–100. - PubMed
-
- Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb Haemost. 1981;45(3):200–203. - PubMed
-
- Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood. 2008;112(2):250–255. [published correction appears in Blood. 2009;113(21):5368] - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

