MAPK-interacting kinase 1 regulates platelet production, activation, and thrombosis
- PMID: 35930749
- PMCID: PMC9918849
- DOI: 10.1182/blood.2022015568
MAPK-interacting kinase 1 regulates platelet production, activation, and thrombosis
Erratum in
-
Manne BK, Campbell RA, Bhatlekar S, et al. MAPK-interacting kinase 1 regulates platelet production, activation, and thrombosis. Blood. 2022;140(23):2477-2489.Blood. 2023 Jun 15;141(24):3007. doi: 10.1182/blood.2023020788. Blood. 2023. PMID: 37318905 Free PMC article. No abstract available.
Abstract
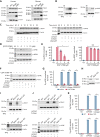
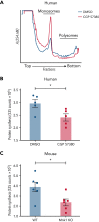
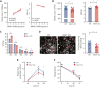
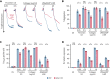
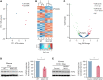
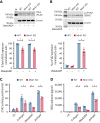
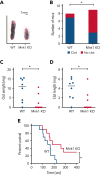
The MAPK-interacting kinase (Mnk) family includes Mnk1 and Mnk2, which are phosphorylated and activated in response to extracellular stimuli. Mnk1 contributes to cellular responses by regulating messenger RNA (mRNA) translation, and mRNA translation influences platelet production and function. However, the role of Mnk1 in megakaryocytes and platelets has not previously been studied. The present study investigated Mnk1 in megakaryocytes and platelets using both pharmacological and genetic approaches. We demonstrate that Mnk1, but not Mnk2, is expressed and active in human and murine megakaryocytes and platelets. Stimulating human and murine megakaryocytes and platelets induced Mnk1 activation and phosphorylation of eIF4E, a downstream target of activated Mnk1 that triggers mRNA translation. Mnk1 inhibition or deletion significantly diminished protein synthesis in megakaryocytes as measured by polysome profiling and [35S]-methionine incorporation assays. Depletion of Mnk1 also reduced megakaryocyte ploidy and proplatelet forming megakaryocytes in vitro and resulted in thrombocytopenia. However, Mnk1 deletion did not affect the half-life of circulating platelets. Platelets from Mnk1 knockout mice exhibited reduced platelet aggregation, α granule secretion, and integrin αIIbβ3 activation. Ribosomal footprint sequencing indicated that Mnk1 regulates the translation of Pla2g4a mRNA (which encodes cPLA2) in megakaryocytes. Consistent with this, Mnk1 ablation reduced cPLA2 activity and thromboxane generation in platelets and megakaryocytes. In vivo, Mnk1 ablation protected against platelet-dependent thromboembolism. These results provide previously unrecognized evidence that Mnk1 regulates mRNA translation and cellular activation in platelets and megakaryocytes, endomitosis and thrombopoiesis, and thrombosis.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.T.R. and J.W.R. disclose that they are coinventors on a patent using platelet transcriptomics. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Novel roles of Mnk1 in megakaryocytes and platelets.Blood. 2022 Dec 8;140(23):2418-2419. doi: 10.1182/blood.2022017936. Blood. 2022. PMID: 36480224 No abstract available.
References
-
- Hartwig J, Italiano J., Jr. The birth of the platelet. J Thromb Haemost. 2003;1(7):1580–1586. - PubMed
-
- Hartwig JH, Italiano JE., Jr. Cytoskeletal mechanisms for platelet production. Blood Cells Mol Dis. 2006;36(2):99–103. - PubMed
-
- Hvas AM. Platelet function in thrombosis and hemostasis. Semin Thromb Hemost. 2016;42(3):183–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

