EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity
- PMID: 35931029
- PMCID: PMC9386785
- DOI: 10.1016/j.stem.2022.06.014
EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity
Erratum in
-
EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity.Cell Stem Cell. 2025 Apr 3;32(4):670. doi: 10.1016/j.stem.2025.03.006. Epub 2025 Mar 14. Cell Stem Cell. 2025. PMID: 40088890 Free PMC article. No abstract available.
Abstract
Human induced pluripotent stem cells (iPSCs) provide a potentially unlimited resource for cell therapies, but the derivation of mature cell types remains challenging. The histone methyltransferase EZH1 is a negative regulator of lymphoid potential during embryonic hematopoiesis. Here, we demonstrate that EZH1 repression facilitates in vitro differentiation and maturation of T cells from iPSCs. Coupling a stroma-free T cell differentiation system with EZH1-knockdown-mediated epigenetic reprogramming, we generated iPSC-derived T cells, termed EZ-T cells, which display a highly diverse T cell receptor (TCR) repertoire and mature molecular signatures similar to those of TCRαβ T cells from peripheral blood. Upon activation, EZ-T cells give rise to effector and memory T cell subsets. When transduced with chimeric antigen receptors (CARs), EZ-T cells exhibit potent antitumor activities in vitro and in xenograft models. Epigenetic remodeling via EZH1 repression allows efficient production of developmentally mature T cells from iPSCs for applications in adoptive cell therapy.
Keywords: CAR T cells; EZH1; T cell differentiation; cancer immunotherapy; hematopoietic stem and progenitor cells; pluripotent stem cells.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.J., G.Q.D., and Boston Children’s Hospital hold intellectual property and receive consulting fees and/or hold equity interest relevant to the generation of iPSC-derived T cells. T.M.S. receives sponsored research support from Elevate Bio. G.Q.D. is a member of Cell Stem Cell’s advisory board.
Figures

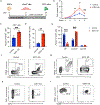

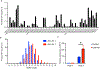

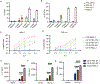

Comment in
-
T cell development made EZ.Cell Stem Cell. 2022 Aug 4;29(8):1155-1156. doi: 10.1016/j.stem.2022.07.003. Cell Stem Cell. 2022. PMID: 35931025
References
-
- Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, and Tamada K (2018) IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol, 36, 346–351. - PubMed
-
- Bosselut R, Kubo S, Guinter T, Kopacz JL, Altman JD, Feigenbaum L, and Singer A (2000) Role of CD8beta domains in CD8 coreceptor function: importance for MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity, 12, 409–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UC4 DK104218/DK/NIDDK NIH HHS/United States
- T32 HL007574/HL/NHLBI NIH HHS/United States
- U01 HL134812/HL/NHLBI NIH HHS/United States
- R13 HL104873/HL/NHLBI NIH HHS/United States
- R01 DK070055/DK/NIDDK NIH HHS/United States
- R01 HL071265/HL/NHLBI NIH HHS/United States
- R01 GM107536/GM/NIGMS NIH HHS/United States
- R24 DK092760/DK/NIDDK NIH HHS/United States
- RC2 DK120535/DK/NIDDK NIH HHS/United States
- RC4 DK090913/DK/NIDDK NIH HHS/United States
- U01 DK104218/DK/NIDDK NIH HHS/United States
- U24 HL134763/HL/NHLBI NIH HHS/United States
- U01 HL100001/HL/NHLBI NIH HHS/United States
- RC2 HL102815/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

