Neurodevelopmental copy-number variants: A roadmap to improving outcomes by uniting patient advocates, researchers, and clinicians for collective impact
- PMID: 35931048
- PMCID: PMC9388383
- DOI: 10.1016/j.ajhg.2022.07.003
Neurodevelopmental copy-number variants: A roadmap to improving outcomes by uniting patient advocates, researchers, and clinicians for collective impact
Abstract
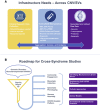
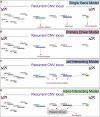
Copy-number variants and structural variants (CNVs/SVs) drive many neurodevelopmental-related disorders. While many neurodevelopmental-related CNVs/SVs give rise to complex phenotypes, the overlap in phenotypic presentation between independent CNVs can be extensive and provides a motivation for shared approaches. This confluence at the level of clinical phenotype implies convergence in at least some aspects of the underlying genomic mechanisms. With this perspective, our Commission on Novel Technologies for Neurodevelopmental CNVs asserts that the time has arrived to approach neurodevelopmental-related CNVs/SVs as a class of disorders that can be identified, investigated, and treated on the basis of shared mechanisms and/or pathways (e.g., molecular, neurological, or developmental). To identify common etiologic mechanisms among uncommon neurodevelopmental-related disorders and to potentially identify common therapies, it is paramount for teams of scientists, clinicians, and patients to unite their efforts. We bring forward novel, collaborative, and integrative strategies to translational CNV/SV research that engages diverse stakeholders to help expedite therapeutic outcomes. We articulate a clear vision for piloted roadmap strategies to reduce patient/caregiver burden and redundancies, increase efficiency, avoid siloed data, and accelerate translational discovery across CNV/SV-based syndromes.
Keywords: CNVs; biobank; community engagement; copy-number variants; genomic disorders; iPSCs; inclusion; infrastructure; long-read sequencing; neurodevelopment; neurological; patient centered; patient led; structural variants; systematic phenotyping; team science.
Copyright © 2022 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Firth H.V., Richards S.M., Bevan A.P., Clayton S., Corpas M., Rajan D., Van Vooren S., Moreau Y., Pettett R.M., Carter N.P. DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. - DOI - PMC - PubMed
-
- Lusk L., Vogel-Farley V., DiStefano C., Jeste S. In: GeneReviews((R)) Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Mirzaa G.M., Amemiya A., editors. 1993. Maternal 15q duplication syndrome. - PubMed

