Extracellular vesicles enriched in connexin 43 promote a senescent phenotype in bone and synovial cells contributing to osteoarthritis progression
- PMID: 35931686
- PMCID: PMC9355945
- DOI: 10.1038/s41419-022-05089-w
Extracellular vesicles enriched in connexin 43 promote a senescent phenotype in bone and synovial cells contributing to osteoarthritis progression
Abstract
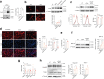
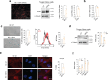
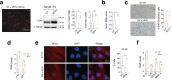
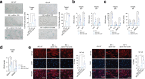
The accumulation of senescent cells is a key characteristic of aging, leading to the progression of age-related diseases such as osteoarthritis (OA). Previous data from our laboratory has demonstrated that high levels of the transmembrane protein connexin 43 (Cx43) are associated with a senescent phenotype in chondrocytes from osteoarthritic cartilage. OA has been reclassified as a musculoskeletal disease characterized by the breakdown of the articular cartilage affecting the whole joint, subchondral bone, synovium, ligaments, tendons and muscles. However, the mechanisms that contribute to the spread of pathogenic factors throughout the joint tissues are still unknown. Here, we show for the first time that small extracellular vesicles (sEVs) released by human OA-derived chondrocytes contain high levels of Cx43 and induce a senescent phenotype in targeted chondrocytes, synovial and bone cells contributing to the formation of an inflammatory and degenerative joint environment by the secretion of senescence-associated secretory associated phenotype (SASP) molecules, including IL-1ß and IL-6 and MMPs. The enrichment of Cx43 changes the protein profile and activity of the secreted sEVs. Our results indicate a dual role for sEVs containing Cx43 inducing senescence and activating cellular plasticity in target cells mediated by NF-kß and the extracellular signal-regulated kinase 1/2 (ERK1/2), inducing epithelial-to-mesenchymal transition (EMT) signalling programme and contributing to the loss of the fully differentiated phenotype. Our results demonstrated that Cx43-sEVs released by OA-derived chondrocytes spread senescence, inflammation and reprogramming factors involved in wound healing failure to neighbouring tissues, contributing to the progression of the disease among cartilage, synovium, and bone and probably from one joint to another. These results highlight the importance for future studies to consider sEVs positive for Cx43 as a new biomarker of disease progression and new target to treat OA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

