Long-Term Efficacy and Safety of Risankizumab in Patients with Active Psoriatic Arthritis: Results from a 76-Week Phase 2 Randomized Trial
- PMID: 35931879
- PMCID: PMC9510089
- DOI: 10.1007/s40744-022-00474-5
Long-Term Efficacy and Safety of Risankizumab in Patients with Active Psoriatic Arthritis: Results from a 76-Week Phase 2 Randomized Trial
Abstract
Introduction: The objective of this work was to assess the efficacy and safety of risankizumab in psoriatic arthritis (PsA) over 76 weeks.
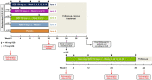
Methods: In this double-blind, dose-ranging phase 2 study, adults with active PsA were randomized 2:2:2:1:2 to risankizumab 150 mg at weeks 0, 4, 8, 12, and 16 (arm 1), 150 mg at weeks 0, 4, and 16 (arm 2), 150 mg at weeks 0 and 12 (arm 3), 75 mg at week 0 (arm 4), or placebo (arm 5). Patients completing week 24 could receive risankizumab 150 mg in a 52-week open-label extension study. Efficacy assessments included American College of Rheumatology (ACR) responses, Psoriasis Area Severity Index (PASI) responses, minimal disease activity (MDA), and 28-joint Disease Activity Score based on C-reactive protein (DAS28[CRP]).
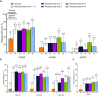
Results: Of 185 randomized patients, 173 (93.5%) completed week 16 and 145 (78.4%) entered the open-label extension. Significantly more patients in each risankizumab arm achieved ACR20 at week 16 versus placebo (primary endpoint: pooled arms 1 + 2 [59.5%] versus placebo [35.7%]; treatment difference [90% CI] 24.0 [9.3, 38.7]; P = 0.007). Similarly, significantly more patients in most risankizumab arms achieved ACR20/50/70, PASI75/90/100, MDA, and greater improvements in DAS28(CRP) versus placebo at week 16. These benefits of risankizumab were maintained long term. Treatment-emergent adverse events were comparable across treatment arms. Risankizumab 150 mg was well tolerated over 76 weeks.
Conclusions: Risankizumab improved joint and skin symptoms versus placebo in patients with active PsA over 16 weeks; improvements were sustained long term. Risankizumab was well tolerated over the long term with no new safety findings.
Trial registration numbers: NCT02719171 and NCT02986373.
Keywords: Interleukins; Psoriatic arthritis; Randomized trial; Risankizumab.
© 2022. The Author(s).
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

