Treatment with FRAX486 rescues neurobehavioral and metabolic alterations in a female mouse model of CDKL5 deficiency disorder
- PMID: 35932179
- PMCID: PMC9532911
- DOI: 10.1111/cns.13907
Treatment with FRAX486 rescues neurobehavioral and metabolic alterations in a female mouse model of CDKL5 deficiency disorder
Abstract
Introduction: CDKL5 deficiency disorder (CDD) is a rare neurodevelopmental condition, primarily affecting girls for which no cure currently exists. Neuronal morphogenesis and plasticity impairments as well as metabolic dysfunctions occur in CDD patients. The present study explored the potential therapeutic value for CDD of FRAX486, a brain-penetrant molecule that was reported to selectively inhibit group I p21-activated kinases (PAKs), serine/threonine kinases critically involved in the regulation of neuronal morphology and glucose homeostasis.
Methods: The effects of treatment with FRAX486 on CDD-related alterations were assessed in vitro (100 nM for 48 h) on primary hippocampal cultures from Cdkl5-knockout male mice (Cdkl5-KO) and in vivo (20 mg/Kg, s.c. for 5 days) on Cdkl5-KO heterozygous females (Cdkl5-Het).
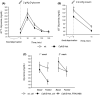
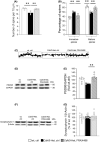
Results: The in vitro treatment with FRAX486 completely rescued the abnormal neuronal maturation and the number of PSD95-positive puncta in Cdkl5-KO mouse neurons. In vivo, FRAX486 normalized the general health status, the hyperactive profile and the fear learning defects of fully symptomatic Cdkl5-Het mice. Systemically, FRAX486 treatment normalized the levels of reactive oxidizing species in the whole blood and the fasting-induced hypoglycemia displayed by Cdkl5-Het mice. In the hippocampus of Cdkl5-Het mice, treatment with FRAX486 rescued spine maturation and PSD95 expression and restored the abnormal PAKs phosphorylation at sites which are critical for their activation (P-PAK-Ser144/141/139) or for the control cytoskeleton remodeling (P-PAK1-Thr212).
Conclusions: Present results provide evidence that PAKs may represent innovative therapeutic targets for CDD.
Keywords: CDKL5 deficiency disorder; animal model; behavior; cytoskeleton; therapeutic approach; transfection.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bahi‐Buisson N, Nectoux J, Rosas‐Vargas H, et al. Key clinical features to identify girls with CDKL5 mutations. Brain J Neurol. 2008;131(Pt 10):2647‐2661. - PubMed
-
- Fehr S, Downs J, Ho G, et al. Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A. 2016;170(11):2860‐2869. - PubMed
-
- Zhu YC, Xiong ZQ. Molecular and synaptic bases of CDKL5 disorder. Dev Neurobiol. 2019;79(1):8‐19. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

