Lack of efficacy of convalescent plasma in COVID-19 patients with concomitant hematological malignancies: An Italian retrospective study
- PMID: 35932208
- PMCID: PMC9538413
- DOI: 10.1002/hon.3060
Lack of efficacy of convalescent plasma in COVID-19 patients with concomitant hematological malignancies: An Italian retrospective study
Abstract
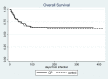
A multicenter retrospective study was designed to assess clinical outcome of COVID-19 in patients with hematological malignancies (HM) following treatment with anti-SARS-CoV-2 convalescent plasma (CP) or standard of care therapy. To this aim, a propensity score matching was used to assess the role of non-randomized administration of CP in this high-risk cohort of patients from the Italian Hematology Alliance on COVID-19 (ITA-HEMA-COV) project, now including 2049 untreated control patients. We investigated 30- and 90-day mortality, rate of admission to intensive care unit, proportion of patients requiring mechanical ventilatory support, hospitalization time, and SARS-CoV-2 clearance in 79 CP recipients and compared results with 158 propensity score-matched controls. Results indicated a lack of efficacy of CP in the study group compared with the untreated group, thus confirming the negative results obtained from randomized studies in immunocompetent individuals with COVID-19. In conclusion, this retrospective analysis did not meet the primary and secondary end points in any category of immunocompromized patients affected by HM.
Keywords: COVID-19; convalescent plasma; disease severity; hematological malignancy; survival data.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
Comment in
-
Convalescent plasma in oncohematological patients.Hematol Oncol. 2023 Feb;41(1):208-209. doi: 10.1002/hon.3069. Epub 2022 Aug 27. Hematol Oncol. 2023. PMID: 36001740 Free PMC article. No abstract available.
References
-
- Seghatchian J, Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID‐19 patients: a rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci. 2020;59(3):102794. 10.1016/j.transci.2020.102794 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous