Association of clinical outcomes and connectivity in awake versus asleep deep brain stimulation for Parkinson disease
- PMID: 35932263
- PMCID: PMC10215008
- DOI: 10.3171/2022.6.JNS212904
Association of clinical outcomes and connectivity in awake versus asleep deep brain stimulation for Parkinson disease
Abstract
Objective: Deep brain stimulation (DBS) for Parkinson disease (PD) is traditionally performed with awake intraoperative testing and/or microelectrode recording. Recently, however, the procedure has been increasingly performed under general anesthesia with image-based verification. The authors sought to compare structural and functional networks engaged by awake and asleep PD-DBS of the subthalamic nucleus (STN) and correlate them with clinical outcomes.
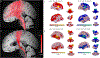
Methods: Levodopa equivalent daily dose (LEDD), pre- and postoperative motor scores on the Movement Disorders Society-Unified Parkinson's Disease Rating Scale part III (MDS-UPDRS III), and total electrical energy delivered (TEED) at 6 months were retroactively assessed in patients with PD who received implants of bilateral DBS leads. In subset analysis, implanted electrodes were reconstructed using the Lead-DBS toolbox. Volumes of tissue activated (VTAs) were used as seed points in group volumetric and connectivity analysis.
Results: The clinical courses of 122 patients (52 asleep, 70 awake) were reviewed. Operating room and procedure times were significantly shorter in asleep cases. LEDD reduction, MDS-UPDRS III score improvement, and TEED at the 6-month follow-up did not differ between groups. In subset analysis (n = 40), proximity of active contact, VTA overlap, and desired network fiber counts with motor STN correlated with lower DBS energy requirement and improved motor scores. Discriminative structural fiber tracts involving supplementary motor area, thalamus, and brainstem were associated with optimal clinical improvement. Areas of highest structural and functional connectivity with VTAs did not significantly differ between the two groups.
Conclusions: Compared to awake STN DBS, asleep procedures can achieve similarly optimal targeting-based on clinical outcomes, electrode placement, and connectivity estimates-in more efficient procedures and shorter operating room times.
Keywords: Parkinson disease; anesthesia; asleep DBS; connectivity; connectomics; deep brain stimulation; functional neurosurgery.
Conflict of interest statement
Figures



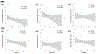
References
-
- Chen T, Mirzadeh Z, Chapple KM, et al. Clinical outcomes following awake and asleep deep brain stimulation for Parkinson disease. J Neurosurg. 2018;130(1):109–120. - PubMed
-
- Mirzadeh Z, Chapple K, Lambert M, et al. Parkinson’s disease outcomes after intraoperative CT-guided “asleep” deep brain stimulation in the globus pallidus internus. J Neurosurg. 2016;124(4):902–907. - PubMed
-
- Moran CH, Pietrzyk M, Sarangmat N, et al. Clinical Outcome of “Asleep” Deep Brain Stimulation for Parkinson Disease Using Robot-Assisted Delivery and Anatomic Targeting of the Subthalamic Nucleus: A Series of 152 Patients. Neurosurgery. 2020;88(1):165–173. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

