Observational cohort study of rilpivirine (RPV) utilization in Europe
- PMID: 35933352
- PMCID: PMC9357334
- DOI: 10.1186/s12981-022-00457-0
Observational cohort study of rilpivirine (RPV) utilization in Europe
Abstract
Introduction: Data on safety and effectiveness of RPV from the real-world setting as well as comparisons with other NNRTIs such as efavirenz (EFV) remain scarce.
Methods: Participants of EuroSIDA were included if they had started a RPV- or an EFV-containing regimen over November 2011-December 2017. Statistical testing was conducted using non-parametric Mann-Whitney U test and Chi-square test. A logistic regression model was used to compare participants' characteristics by treatment group. Kaplan-Meier analysis was used to estimate the cumulative risk of virological failure (VF, two consecutive values > 50 copies/mL).
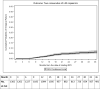
Results: 1,355 PLWH who started a RPV-based regimen (11% ART-naïve), as well as 333 initiating an EFV-containing regimen were included. Participants who started RPV differed from those starting EFV for demographics (age, geographical region) and immune-virological profiles (CD4 count, HIV RNA). The cumulative risk of VF for the RPV-based group was 4.5% (95% CI 3.3-5.7%) by 2 years from starting treatment (71 total VF events). Five out of 15 (33%) with resistance data available in the RPV group showed resistance-associated mutations vs. 3/13 (23%) among those in the EFV group. Discontinuations due to intolerance/toxicity were reported for 73 (15%) of RPV- vs. 45 (30%) of EFV-treated participants (p = 0.0001). The main difference was for toxicity of central nervous system (CNS, 3% vs. 22%, p < 0.001).
Conclusion: Our estimates of VF > 50 copies/mL and resistance in participants treated with RPV were similar to those reported by other studies. RPV safety profile was favourable with less frequent discontinuation due to toxicity than EFV (especially for CNS).
Keywords: Cohort Study; Edurant™; Efavirenz; Europe; Eviplera™; Real-world effectiveness.
© 2022. The Author(s).
Conflict of interest statement
This analysis was funded by Janssen Research and Development, who did not influence the analyses presented or the decision to publish study findings. Amanda Mocroft received honoraria, travel support, lecture fees and consultancy for ViiV, Gilead, Eiland and Bonnin, all outside the submitted work.
Figures
References
-
- EACS European Guidelines for treatment of HIV-positive adults in Europe. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
-
- Pozniak AL, Morales-Ramirez J, Katabira E, Steyn D, Lupo SH, Santoscoy M, Grinsztejn B, Ruxrungtham K, Rimsky LT, Vanveggel S, Boven K. TMC278-C204 Study Group. Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS. 2010;24(1):55–65. doi: 10.1097/QAD.0b013e32833032ed. - DOI - PubMed
-
- Nelson MR, Elion RA, Cohen CJ, Mills A, Hodder SL, Segal-Maurer S, Bloch M, Garner W, Guyer B, Williams S, Chuck S, Vanveggel S, Deckx H, Stevens M. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE Studies. HIV Clin Trials. 2013;14(3):81–91. doi: 10.1310/hct1403-81. - DOI - PubMed
-
- Mills AM, Cohen C, Dejesus E, Brinson C, Williams S, Yale KL, Ramanathan S, Wang MH, White K, Chuck SK, Cheng AK. Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. HIV Clin Trials. 2013;14(5):216–223. doi: 10.1310/hct1405-216. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

