What is the best technic to dislodge Staphylococcus epidermidis biofilm on medical implants?
- PMID: 35933363
- PMCID: PMC9356421
- DOI: 10.1186/s12866-022-02606-x
What is the best technic to dislodge Staphylococcus epidermidis biofilm on medical implants?
Abstract
Background: Bacterial biofilm can occur on all medical implanted devices and lead to infection and/or dysfunction of the device. In this study, artificial biofilm was formed on four different medical implants (silicone, piccline, peripheral venous catheter and endotracheal tube) of interest for our daily clinical and/or research practice. We investigated the best conventional technic to dislodge the biofilm on the implants and quantified the number of bacteria. Staphylococcus epidermidis previously isolated from a breast implant capsular contracture on a patient in the university hospital of Dijon was selected for its ability to produce biofilm on the implants. Different technics (sonication, Digest-EUR®, mechanized bead mill, combination of sonication plus Digest-EUR®) were tested and compared to detach the biofilm before quantifying viable bacteria by colony counting.
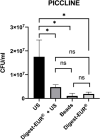
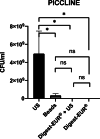
Results: For all treatments, the optical and scanning electron microscope images showed substantial less biofilm biomass remaining on the silicone implant compared to non-treated implant. This study demonstrated that the US procedure was statistically superior to the other physical treatment: beads, Digest-EUR® alone and Digest-EUR® + US (p < 0.001) for the flexible materials (picc-line, PIV, and silicone). The number of bacteria released by the US is significantly higher with a difference of 1 log on each material. The result for a rigid endotracheal tube were different with superiority for the chemical treatment dithiothreitol: Digest-EUR®. Surprisingly the combination of the US plus Digest-EUR® treatment was consistently inferior for the four materials.
Conclusions: Depending on the materials used, the biofilm dislodging technique must be adapted. The US procedure was the best technic to dislodge S. epidermidis biofilm on silicone, piccline, peripheral venous catheter but not endotracheal tube. This suggested that scientists should compare themselves different methods before designing a protocol of biofilm study on a given material.
Keywords: Biofilm quantification; Endotracheal tube; Enzymatic treatment; Medical implant; Peripheral venous catheter; Piccline; Silicone; Sonication; Staphylococcus epidermidis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









Similar articles
-
Comparison of sonication with chemical biofilm dislodgement methods using chelating and reducing agents: Implications for the microbiological diagnosis of implant associated infection.PLoS One. 2020 Apr 8;15(4):e0231389. doi: 10.1371/journal.pone.0231389. eCollection 2020. PLoS One. 2020. PMID: 32267888 Free PMC article.
-
[In vivo study on the effects of intercellular adhesion operon of Staphylococcus epidermidis on the inflammation associated with bacteria-fungal mixed biofilm].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 Oct 15;35(10):1328-1335. doi: 10.7507/1002-1892.202104061. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021. PMID: 34651489 Free PMC article. Chinese.
-
Highly variable effect of sonication to dislodge biofilm-embedded Staphylococcus epidermidis directly quantified by epifluorescence microscopy: an in vitro model study.J Orthop Surg Res. 2020 Nov 11;15(1):522. doi: 10.1186/s13018-020-02052-3. J Orthop Surg Res. 2020. PMID: 33176843 Free PMC article.
-
Staphylococcus aureus and Staphylococcus epidermidis infections on implants.J Hosp Infect. 2018 Feb;98(2):111-117. doi: 10.1016/j.jhin.2017.11.008. Epub 2017 Nov 22. J Hosp Infect. 2018. PMID: 29175074 Review.
-
A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants.Curr Res Microb Sci. 2024 Mar 7;6:100231. doi: 10.1016/j.crmicr.2024.100231. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 38510214 Free PMC article. Review.
Cited by
-
In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite.J Funct Biomater. 2025 Feb 16;16(2):66. doi: 10.3390/jfb16020066. J Funct Biomater. 2025. PMID: 39997600 Free PMC article.
-
Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review.Antibiotics (Basel). 2024 Jul 22;13(7):678. doi: 10.3390/antibiotics13070678. Antibiotics (Basel). 2024. PMID: 39061360 Free PMC article. Review.
-
Sorbate metal complexes as newer antibacterial, antibiofilm, and anticancer compounds.BMC Microbiol. 2024 Jul 18;24(1):262. doi: 10.1186/s12866-024-03370-w. BMC Microbiol. 2024. PMID: 39026170 Free PMC article.
-
Formation of Single-Species and Multispecies Biofilm by Isolates from Septic Transfusion Reactions in Platelet Bag Model.Emerg Infect Dis. 2024 Sep;30(9):1819-1828. doi: 10.3201/eid3009.240372. Epub 2024 Aug 6. Emerg Infect Dis. 2024. PMID: 39106464 Free PMC article.
-
Comparative evaluation of proteinase K and dithiothreitol as pretreatments for extracting nucleic acids from respiratory samples for multiplex PCR.BMC Microbiol. 2025 Apr 23;25(1):233. doi: 10.1186/s12866-025-03956-y. BMC Microbiol. 2025. PMID: 40263996 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

