GREM1/PPP2R3A expression in heterogeneous fibroblasts initiates pulmonary fibrosis
- PMID: 35933397
- PMCID: PMC9356444
- DOI: 10.1186/s13578-022-00860-0
GREM1/PPP2R3A expression in heterogeneous fibroblasts initiates pulmonary fibrosis
Abstract
Background: Fibroblasts have important roles in the synthesis and remodeling of extracellular matrix (ECM) proteins during pulmonary fibrosis. However, the spatiotemporal distribution of heterogeneous fibroblasts during disease progression remains unknown.
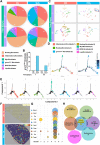
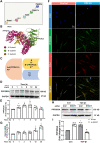
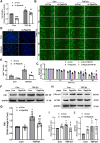
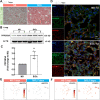
Results: In the current study, silica was used to generate a mouse model of pathological changes in the lung, and single-cell sequencing, spatial transcriptome sequencing and an analysis of markers of cell subtypes were performed to identify fibroblast subtypes. A group of heterogeneous fibroblasts that play an important role at the early pathological stage were identified, characterized based on the expression of inflammatory and proliferation genes (termed inflammatory-proliferative fibroblasts) and found to be concentrated in the lesion area. The expression of GREM1/protein phosphatase 2 regulatory subunit B''alpha (PPP2R3A) in inflammatory-proliferative fibroblasts was found to initiate early pulmonary pathological changes by increasing the viability, proliferation and migration of cells.
Conclusions: Inflammatory-proliferative fibroblasts play a key role in the early pathological changes that occur in silicosis, and during this process, GREM1 is the driving factor that targets PPP2R3A and initiates the inflammatory response, which is followed by irreversible fibrosis induced by SiO2. The GREM1/PPP2R3A pathway may be a potential target in the early treatment of silicosis.
Keywords: Heterogeneous fibroblasts; Pulmonary fibrosis; Single-cell transcriptomics; Spatial transcriptomics.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Protein phosphatase 2 regulatory subunit B''Alpha silencing inhibits tumor cell proliferation in liver cancer.Cancer Med. 2019 Dec;8(18):7741-7753. doi: 10.1002/cam4.2620. Epub 2019 Oct 24. Cancer Med. 2019. PMID: 31647192 Free PMC article.
-
circHIPK2-mediated σ-1R promotes endoplasmic reticulum stress in human pulmonary fibroblasts exposed to silica.Cell Death Dis. 2017 Dec 13;8(12):3212. doi: 10.1038/s41419-017-0017-4. Cell Death Dis. 2017. PMID: 29238093 Free PMC article.
-
Role of human pulmonary fibroblast-derived MCP-1 in cell activation and migration in experimental silicosis.Toxicol Appl Pharmacol. 2015 Oct 15;288(2):152-60. doi: 10.1016/j.taap.2015.07.002. Epub 2015 Jul 8. Toxicol Appl Pharmacol. 2015. PMID: 26163174
-
[Overexpression of protein phosphatase 2 regulatory subunit B''α gene effect on proliferation and invasion of hepatoma cells].Zhonghua Gan Zang Bing Za Zhi. 2019 Nov 20;27(11):872-878. doi: 10.3760/cma.j.issn.1007-3418.2019.11.010. Zhonghua Gan Zang Bing Za Zhi. 2019. PMID: 31941242 Chinese.
-
Autophagy, an important therapeutic target for pulmonary fibrosis diseases.Clin Chim Acta. 2020 Mar;502:139-147. doi: 10.1016/j.cca.2019.12.016. Epub 2019 Dec 23. Clin Chim Acta. 2020. PMID: 31877297 Review.
Cited by
-
The protein phosphatase-2A subunit PR130 is involved in the formation of cytotoxic protein aggregates in pancreatic ductal adenocarcinoma cells.Cell Commun Signal. 2024 Apr 3;22(1):217. doi: 10.1186/s12964-024-01597-8. Cell Commun Signal. 2024. PMID: 38570831 Free PMC article.
-
Hypercontractile cardiac phenotype in mice overexpressing the regulatory subunit PR72 of protein phosphatase 2A.Front Cardiovasc Med. 2023 Oct 6;10:1239555. doi: 10.3389/fcvm.2023.1239555. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37868783 Free PMC article.
-
Spatial transcriptomics: recent developments and insights in respiratory research.Mil Med Res. 2023 Aug 17;10(1):38. doi: 10.1186/s40779-023-00471-x. Mil Med Res. 2023. PMID: 37592342 Free PMC article. Review.
-
Demethyleneberberine Alleviates Pulmonary Fibrosis through Disruption of USP11 Deubiquitinating GREM1.Pharmaceuticals (Basel). 2024 Feb 22;17(3):279. doi: 10.3390/ph17030279. Pharmaceuticals (Basel). 2024. PMID: 38543064 Free PMC article.
-
Shared genetic correlations between kidney diseases and sepsis.Front Endocrinol (Lausanne). 2024 Jul 17;15:1396041. doi: 10.3389/fendo.2024.1396041. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39086896 Free PMC article.
References
-
- Alvarez RF, Gonzalez CM, Martinez AQ, Perez JJB, Fernandez LC, Fernandez AP. Guidelines for the diagnosis and monitoring of silicosis. Arch Bronconeumol. 2015;51(2):86–93. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

