Does persistence to methotrexate treatment in early rheumatoid arthritis have a familial component?
- PMID: 35933427
- PMCID: PMC9356456
- DOI: 10.1186/s13075-022-02873-z
Does persistence to methotrexate treatment in early rheumatoid arthritis have a familial component?
Abstract
Objectives: To assess whether persistence to treatment with methotrexate (MTX) in early rheumatoid arthritis (RA) is shared among first-degree relatives with RA and to estimate any underlying heritability.
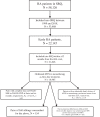
Methods: First-degree relative pairs diagnosed with RA 1999-2018 and starting MTX (in monotherapy) as their first disease-modifying anti-rheumatic drug (DMARD) treatment were identified by linking the Swedish Rheumatology Quality Register to national registers. Short- and long-term persistence to MTX was defined as remaining on treatment at 1 and 3 years, respectively, with no additional DMARDs added. We assessed familial aggregation through relative risks (RR) using log-binomial regression with robust standard errors and estimated heritability using tetrachoric correlations. We also explored the familial aggregation of EULAR treatment response after 3 and 6 months. To mimic the clinical setting, we also tested the association between having a family history of MTX persistence and persistence within the index patient.
Results: Familial persistence was not associated with persistence at 1 (RR=1.02, 95% CI 0.87-1.20), only at 3 (RR=1.41, 95% CI 1.14-1.74) years. Heritability at 1 and 3 years was estimated to be 0.08 (95% CI 0-0.43) and 0.58 (95% CI 0.27-0.89), respectively. No significant associations were found between family history and EULAR response at 3 and 6 months, neither overall nor in the clinical setting analysis.
Conclusions: Our findings imply a familial component, including a possible genetic element, within the long-term persistence to MTX following RA diagnosis. Whether this component is reflective of characteristics of the underlying RA disease or determinants for sustained response to MTX in itself will require further investigation.
Keywords: Familiality; Heritability; Methotrexate; Rheumatoid arthritis; Treatment persistence.
© 2022. The Author(s).
Conflict of interest statement
Karolinska Institutet has entered into agreements with the following companies, with JA as PI: Abbvie, BMS, Eli Lilly, Janssen, Pfizer, Roche, Samsung Bioepis, and Sanofi. SS is a part-time employee as a researcher at deCODE genetics Inc.
Figures
Similar articles
-
Genome-wide investigation of persistence with methotrexate treatment in early rheumatoid arthritis.Rheumatology (Oxford). 2024 May 2;63(5):1221-1229. doi: 10.1093/rheumatology/kead301. Rheumatology (Oxford). 2024. PMID: 37326842 Free PMC article.
-
Does a family history of RA influence the clinical presentation and treatment response in RA?Ann Rheum Dis. 2016 Jun;75(6):1120-5. doi: 10.1136/annrheumdis-2015-207670. Epub 2015 Jun 19. Ann Rheum Dis. 2016. PMID: 26091906
-
[Re-recognition of methotrexate monotherapy in rheumatoid arthritis].Zhonghua Yi Xue Za Zhi. 2022 Oct 11;102(37):2909-2913. doi: 10.3760/cma.j.cn112137-20220224-00394. Zhonghua Yi Xue Za Zhi. 2022. PMID: 36207865 Chinese.
-
Tofacitinib for Treating Rheumatoid Arthritis After the Failure of Disease-Modifying Anti-rheumatic Drugs: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Sep;36(9):1063-1072. doi: 10.1007/s40273-018-0639-0. Pharmacoeconomics. 2018. PMID: 29546668 Review.
-
Meta-Regression of a Dose-Response Relationship of Methotrexate in Mono- and Combination Therapy in Disease-Modifying Antirheumatic Drug-Naive Early Rheumatoid Arthritis Patients.Arthritis Care Res (Hoboken). 2017 Oct;69(10):1473-1483. doi: 10.1002/acr.23164. Epub 2017 Aug 31. Arthritis Care Res (Hoboken). 2017. PMID: 27992656 Review.
Cited by
-
Genome-wide investigation of persistence with methotrexate treatment in early rheumatoid arthritis.Rheumatology (Oxford). 2024 May 2;63(5):1221-1229. doi: 10.1093/rheumatology/kead301. Rheumatology (Oxford). 2024. PMID: 37326842 Free PMC article.
-
Common genetic variants do not impact clinical prediction of methotrexate treatment outcomes in early rheumatoid arthritis.J Intern Med. 2025 Jun;297(6):693-701. doi: 10.1111/joim.20087. Epub 2025 Apr 6. J Intern Med. 2025. PMID: 40190030 Free PMC article.
References
-
- Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheum. 2016;68(1):1–26. - PubMed
-
- Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
-
- Braun J, Kastner P, Flaxenberg P, Wahrisch J, Hanke P, Demary W, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum. 2008;58(1):73–81. doi: 10.1002/art.23144. - DOI - PubMed
-
- Saevarsdottir S, Wallin H, Seddighzadeh M, Ernestam S, Geborek P, Petersson IF, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70(3):469–475. doi: 10.1136/ard.2010.139212. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

