Possible mediators of metabolic endotoxemia in women with obesity and women with obesity-diabetes in The Gambia
- PMID: 35933445
- PMCID: PMC9492538
- DOI: 10.1038/s41366-022-01193-1
Possible mediators of metabolic endotoxemia in women with obesity and women with obesity-diabetes in The Gambia
Abstract
Aims/hypothesis: Translocation of bacterial debris from the gut causes metabolic endotoxemia (ME) that results in insulin resistance, and may be on the causal pathway to obesity-related type 2 diabetes. To guide interventions against ME we tested two hypothesised mechanisms for lipopolysaccharide (LPS) ingress: a leaky gut and chylomicron-associated transfer following a high-fat meal.
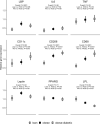
Methods: In lean women (n = 48; fat mass index (FMI) 9.6 kg/m2), women with obesity (n = 62; FMI 23.6 kg/m2) and women with obesity-diabetes (n = 38; FMI 24.9 kg/m2) we used the lactulose-mannitol dual-sugar permeability test (LM ratio) to assess gut integrity. Markers of ME (LPS, EndoCAb IgG and IgM, IL-6, CD14 and lipoprotein binding protein) were assessed at baseline, 2 h and 5 h after a standardised 49 g fat-containing mixed meal. mRNA expression of markers of inflammation, macrophage activation and lipid metabolism were measured in peri-umbilical adipose tissue (AT) biopsies.
Results: The LM ratio did not differ between groups. LPS levels were 57% higher in the obesity-diabetes group (P < 0.001), but, contrary to the chylomicron transfer hypothesis, levels significantly declined following the high-fat challenge. EndoCAb IgM was markedly lower in women with obesity and women with obesity-diabetes. mRNA levels of inflammatory markers in adipose tissue were consistent with the prior concept that fat soluble LPS in AT attracts and activates macrophages.
Conclusions/interpretation: Raised levels of LPS and IL-6 in women with obesity-diabetes and evidence of macrophage activation in adipose tissue support the concept of metabolic endotoxemia-mediated inflammation, but we found no evidence for abnormal gut permeability or chylomicron-associated post-prandial translocation of LPS. Instead, the markedly lower EndoCAb IgM levels indicate a failure in sequestration and detoxification.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

