Atmospheric chemosynthesis is phylogenetically and geographically widespread and contributes significantly to carbon fixation throughout cold deserts
- PMID: 35933499
- PMCID: PMC9561532
- DOI: 10.1038/s41396-022-01298-5
Atmospheric chemosynthesis is phylogenetically and geographically widespread and contributes significantly to carbon fixation throughout cold deserts
Abstract
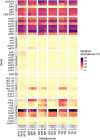
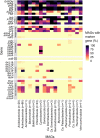
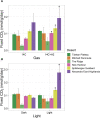
Cold desert soil microbiomes thrive despite severe moisture and nutrient limitations. In Eastern Antarctic soils, bacterial primary production is supported by trace gas oxidation and the light-independent RuBisCO form IE. This study aims to determine if atmospheric chemosynthesis is widespread within Antarctic, Arctic and Tibetan cold deserts, to identify the breadth of trace gas chemosynthetic taxa and to further characterize the genetic determinants of this process. H2 oxidation was ubiquitous, far exceeding rates reported to fulfill the maintenance needs of similarly structured edaphic microbiomes. Atmospheric chemosynthesis occurred globally, contributing significantly (p < 0.05) to carbon fixation in Antarctica and the high Arctic. Taxonomic and functional analyses were performed upon 18 cold desert metagenomes, 230 dereplicated medium-to-high-quality derived metagenome-assembled genomes (MAGs) and an additional 24,080 publicly available genomes. Hydrogenotrophic and carboxydotrophic growth markers were widespread. RuBisCO IE was discovered to co-occur alongside trace gas oxidation enzymes in representative Chloroflexota, Firmicutes, Deinococcota and Verrucomicrobiota genomes. We identify a novel group of high-affinity [NiFe]-hydrogenases, group 1m, through phylogenetics, gene structure analysis and homology modeling, and reveal substantial genetic diversity within RuBisCO form IE (rbcL1E), and high-affinity 1h and 1l [NiFe]-hydrogenase groups. We conclude that atmospheric chemosynthesis is a globally-distributed phenomenon, extending throughout cold deserts, with significant implications for the global carbon cycle and bacterial survival within environmental reservoirs.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Soil Microbiomes With the Genetic Capacity for Atmospheric Chemosynthesis Are Widespread Across the Poles and Are Associated With Moisture, Carbon, and Nitrogen Limitation.Front Microbiol. 2020 Aug 12;11:1936. doi: 10.3389/fmicb.2020.01936. eCollection 2020. Front Microbiol. 2020. PMID: 32903524 Free PMC article.
-
Atmospheric trace gases support primary production in Antarctic desert surface soil.Nature. 2017 Dec 21;552(7685):400-403. doi: 10.1038/nature25014. Epub 2017 Dec 6. Nature. 2017. PMID: 29211716
-
Persistence and resistance: survival mechanisms of Candidatus Dormibacterota from nutrient-poor Antarctic soils.Environ Microbiol. 2021 Aug;23(8):4276-4294. doi: 10.1111/1462-2920.15610. Epub 2021 Jun 3. Environ Microbiol. 2021. PMID: 34029441
-
Clearing the air: unraveling past and guiding future research in atmospheric chemosynthesis.Microbiol Mol Biol Rev. 2023 Dec 20;87(4):e0004823. doi: 10.1128/mmbr.00048-23. Epub 2023 Nov 1. Microbiol Mol Biol Rev. 2023. PMID: 37914532 Free PMC article. Review.
-
Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO).Microbiol Rev. 1996 Dec;60(4):609-40. doi: 10.1128/mr.60.4.609-640.1996. Microbiol Rev. 1996. PMID: 8987358 Free PMC article. Review.
Cited by
-
The Vestfold Hills are alive: characterising microbial and environmental dynamics in Old Wallow, eastern Antarctica.Front Microbiol. 2024 Sep 23;15:1443491. doi: 10.3389/fmicb.2024.1443491. eCollection 2024. Front Microbiol. 2024. PMID: 39376700 Free PMC article.
-
Impact of Temperature Elevation on Microbial Communities and Antibiotic Degradation in Cold Region Soils of Northeast China.Toxics. 2024 Sep 13;12(9):667. doi: 10.3390/toxics12090667. Toxics. 2024. PMID: 39330595 Free PMC article.
-
Genome-resolved metagenomics identifies novel active microbes in biogeochemical cycling within methanol-enriched soil.Environ Microbiol Rep. 2024 Apr;16(2):e13246. doi: 10.1111/1758-2229.13246. Environ Microbiol Rep. 2024. PMID: 38575138 Free PMC article.
-
Geology defines microbiome structure and composition in nunataks and valleys of the Sør Rondane Mountains, East Antarctica.Front Microbiol. 2024 Feb 6;15:1316633. doi: 10.3389/fmicb.2024.1316633. eCollection 2024. Front Microbiol. 2024. PMID: 38380088 Free PMC article.
-
Novel endolithic bacteria of phylum Chloroflexota reveal a myriad of potential survival strategies in the Antarctic desert.Appl Environ Microbiol. 2024 Mar 20;90(3):e0226423. doi: 10.1128/aem.02264-23. Epub 2024 Feb 19. Appl Environ Microbiol. 2024. PMID: 38372512 Free PMC article.
References
-
- Kleinteich J, Hildebrand F, Bahram M, Voigt AY, Wood SA, Jungblut AD, et al. Pole-to-pole connections: similarities between Arctic and Antarctic microbiomes and their vulnerability to environmental change. Front Ecol Evol. 2017;5.

