Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis
- PMID: 35933512
- PMCID: PMC9357088
- DOI: 10.1038/s41467-022-32325-w
Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis
Abstract
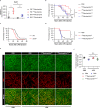
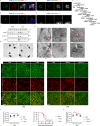
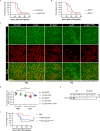
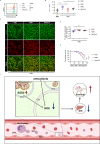
Disseminated intravascular coagulation (DIC) is a complication of sepsis currently lacking effective therapeutic options. Excessive inflammatory responses are emerging triggers of coagulopathy during sepsis, but the interplay between the immune system and coagulation are not fully understood. Here we utilize a murine model of intraperitoneal lipopolysaccharide stimulation and show neutrophils in the circulation mitigate the occurrence of DIC, preventing subsequent septic death. We show circulating neutrophils release extracellular vesicles containing mitochondria, which contain superoxide dismutase 2 upon exposure to lipopolysaccharide. Extracellular superoxide dismutase 2 is necessary to induce neutrophils' antithrombotic function by preventing endothelial reactive oxygen species accumulation and alleviating endothelial dysfunction. Intervening endothelial reactive oxygen species accumulation by antioxidants significantly ameliorates disseminated intravascular coagulation improving survival in this murine model of lipopolysaccharide challenge. These findings reveal an interaction between neutrophils and vascular endothelium which critically regulate coagulation in a model of sepsis and may have potential implications for the management of disseminated intravascular coagulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ultramicronized Palmitoylethanolamide in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation.Int J Mol Sci. 2021 Oct 21;22(21):11388. doi: 10.3390/ijms222111388. Int J Mol Sci. 2021. PMID: 34768820 Free PMC article.
-
Recent advances in the research and management of sepsis-associated DIC.Int J Hematol. 2021 Jan;113(1):24-33. doi: 10.1007/s12185-020-03053-y. Epub 2021 Jan 2. Int J Hematol. 2021. PMID: 33386597 Free PMC article. Review.
-
Embelin ameliorated sepsis-induced disseminated intravascular coagulation intensities by simultaneously suppressing inflammation and thrombosis.Biomed Pharmacother. 2020 Oct;130:110528. doi: 10.1016/j.biopha.2020.110528. Epub 2020 Jul 20. Biomed Pharmacother. 2020. PMID: 32702634
-
Bacteria-released outer membrane vesicles promote disseminated intravascular coagulation.Thromb Res. 2019 Jun;178:26-33. doi: 10.1016/j.thromres.2019.03.019. Epub 2019 Mar 28. Thromb Res. 2019. PMID: 30953960
-
Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation.Semin Thromb Hemost. 2020 Feb;46(1):89-95. doi: 10.1055/s-0039-1694995. Epub 2019 Aug 23. Semin Thromb Hemost. 2020. PMID: 31443111 Review.
Cited by
-
Migrasomes, a new mode of intercellular communication.Cell Commun Signal. 2023 May 8;21(1):105. doi: 10.1186/s12964-023-01121-4. Cell Commun Signal. 2023. PMID: 37158915 Free PMC article. Review.
-
Pathogenic and therapeutic roles of extracellular vesicles in sepsis.Front Immunol. 2025 Feb 4;16:1535427. doi: 10.3389/fimmu.2025.1535427. eCollection 2025. Front Immunol. 2025. PMID: 39967672 Free PMC article. Review.
-
Cathelicidin-HG Alleviates Sepsis-Induced Platelet Dysfunction by Inhibiting GPVI-Mediated Platelet Activation.Research (Wash D C). 2024 Jun 5;7:0381. doi: 10.34133/research.0381. eCollection 2024. Research (Wash D C). 2024. PMID: 38840901 Free PMC article.
-
Differential neutrophil responses in murine following intraperitoneal injections of Escherichia coli and Staphylococcus aureus.Heliyon. 2024 Nov 15;10(22):e40281. doi: 10.1016/j.heliyon.2024.e40281. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39641065 Free PMC article.
-
Superoxide dismutase alterations in COVID-19: implications for disease severity and mortality prediction in the context of omicron variant infection.Front Immunol. 2024 Feb 22;15:1362102. doi: 10.3389/fimmu.2024.1362102. eCollection 2024. Front Immunol. 2024. PMID: 38464514 Free PMC article.
References
-
- Giustozzi, M. et al. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev., 100864, 10.1016/j.blre.2021.100864 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

