Unraveling the effect of intra- and intercellular processes on acetaminophen-induced liver injury
- PMID: 35933513
- PMCID: PMC9357019
- DOI: 10.1038/s41540-022-00238-5
Unraveling the effect of intra- and intercellular processes on acetaminophen-induced liver injury
Abstract
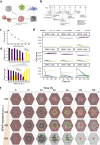
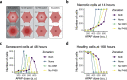
In high dosages, acetaminophen (APAP) can cause severe liver damage, but susceptibility to liver failure varies across individuals and is influenced by factors such as health status. Because APAP-induced liver injury and recovery is regulated by an intricate system of intra- and extracellular molecular signaling, we here aim to quantify the importance of specific modules in determining the outcome after an APAP insult and of potential targets for therapies that mitigate adversity. For this purpose, we integrated hepatocellular acetaminophen metabolism, DNA damage response induction and cell fate into a multiscale mechanistic liver lobule model which involves various cell types, such as hepatocytes, residential Kupffer cells and macrophages. Our model simulations show that zonal differences in metabolism and detoxification efficiency are essential determinants of necrotic damage. Moreover, the extent of senescence, which is regulated by intracellular processes and triggered by extracellular signaling, influences the potential to recover. In silico therapies at early and late time points after APAP insult indicated that prevention of necrotic damage is most beneficial for recovery, whereas interference with regulation of senescence promotes regeneration in a less pronounced way.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Sheng Y, Liang Q, Deng Z, Ji L, Wang Z. Acetaminophen induced gender-dependent liver injury and the involvement of GCL and GPx. Drug Discov. Ther. 2013;7:78–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

