The minimal FLASH sparing effect needed to compensate the increase of radiobiological damage due to hypofractionation for late-reacting tissues
- PMID: 35933554
- PMCID: PMC10087769
- DOI: 10.1002/mp.15911
The minimal FLASH sparing effect needed to compensate the increase of radiobiological damage due to hypofractionation for late-reacting tissues
Abstract
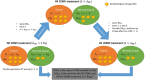
Purpose: Normal tissue (NT) sparing by ultra-high dose rate (UHDR) irradiations compared to conventional dose rate (CONV) irradiations while being isotoxic to the tumor has been termed "FLASH effect" and has been observed when large doses per fraction (d ≳ 5 Gy) have been delivered. Since hypofractionated treatment schedules are known to increase toxicities of late-reacting tissues compared to normofractionated schedules for many clinical scenarios at CONV dose rates, we developed a formalism based on the biologically effective dose (BED) to assess the minimum magnitude of the FLASH effect needed to compensate the loss of late-reacting NT sparing when reducing the number of fractions compared to a normofractionated CONV treatment schedule while remaining isoeffective to the tumor.
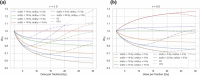
Methods: By requiring the same BED for the tumor, we derived the "break-even NT sparing weighting factor" WBE for the linear-quadratic (LQ) and LQ-linear (LQ-L) models for an NT region irradiated at a relative dose r (relative to the prescribed dose per fraction d to the tumor). WBE was evaluated numerically for multiple values of d and r, and for different tumor and NT α/β-ratios. WBE was compared against currently available experimental data on the magnitude of the NT sparing provided by the FLASH effect for single fraction doses.
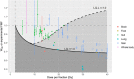
Results: For many clinically relevant scenarios, WBE decreases steeply initially for d > 2 Gy for late-reacting tissues with (α/β)NT ≈ 3 Gy, implying that a significant NT sparing by the FLASH effect (between 15% and 30%) is required to counteract the increased radiobiological damage experienced by late-reacting NT for hypofractionated treatments with d < 10 Gy compared to normofractionated treatments that are equieffective to the tumor. When using the LQ model with generic α/β-ratios for tumor and late-reacting NT of (α/β)T = 10 Gy and (α/β)NT = 3 Gy, respectively, most currently available experimental evidence about the magnitude of NT sparing by the FLASH effect suggests no net NT sparing benefit for hypofractionated FLASH radiotherapy (RT) in the high-dose region when compared with WBE . Instead, clinical indications with more similar α/β-ratios of the tumor and dose-limiting NT toxicities [i.e., (α/β)T ≈ (α/β)NT ], such as prostate treatments, are generally less penalized by hypofractionated treatments and need consequently smaller magnitudes of NT sparing by the FLASH effect to achieve a net benefit. For strongly hypofractionated treatments (>10-15 Gy/fraction), the LQ-L model predicts, unlike the LQ model, a larger WBE suggesting a possible benefit of strongly hypofractionated FLASH RT, even for generic α/β-ratios of (α/β)T = 10 Gy and (α/β)NT = 3 Gy. However, knowledge on the isoeffect scaling for high doses per fraction (≳10 Gy/fraction) and its modeling is currently limited and impedes accurate and reliable predictions for such strongly hypofractionated treatments.
Conclusions: We developed a formalism that quantifies the minimal NT sparing by the FLASH effect needed to compensate for hypofractionation, based on the LQ and LQ-L models. For a given hypofractionated UHDR treatment scenario and magnitude of the FLASH effect, the formalism predicts if a net NT sparing benefit is expected compared to a respective normofractionated CONV treatment.
Keywords: BED; FLASH effect; LQ model; LQ-L model; hypofractionation.
© 2022 The Authors. Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

References
-
- Rosenthal DI, Glatstein E. We've got a treatment, but what's the disease? Or a brief history of hypofractionation and its relationship to stereotactic radiosurgery. Oncologist. 1996;1(1‐2):1‐7. - PubMed
-
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 8th ed. Philadelphia: Wolters Kluwer; 2019.
-
- ICRU . ICRU Report 91: prescribing, recording, and reporting of stereotactic treatments with small photon beams. J ICRU. 2014;14(2). doi:10.1093/jicru/ndx006 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

