Elevation of gangliosides in four brain regions from Parkinson's disease patients with a GBA mutation
- PMID: 35933559
- PMCID: PMC9357011
- DOI: 10.1038/s41531-022-00363-2
Elevation of gangliosides in four brain regions from Parkinson's disease patients with a GBA mutation
Abstract
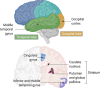
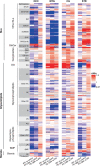
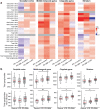
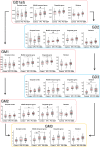
A number of genetic risk factors have been identified over the past decade for Parkinson's Disease (PD), with variants in GBA prominent among them. GBA encodes the lysosomal enzyme that degrades the glycosphingolipid, glucosylceramide (GlcCer), with the activity of this enzyme defective in Gaucher disease. Based on the ill-defined relationship between glycosphingolipid metabolism and PD, we now analyze levels of various lipids by liquid chromatography/electrospray ionization-tandem mass spectrometry in four brain regions from age- and sex-matched patient samples, including idiopathic PD, PD patients with a GBA mutation and compare both to control brains (n = 21 for each group) obtained from individuals who died from a cause unrelated to PD. Of all the glycerolipids, sterols, and (glyco)sphingolipids (251 lipids in total), the only lipid class which showed significant differences were the gangliosides (sialic acid-containing complex glycosphingolipids), which were elevated in 3 of the 4 PD-GBA brain regions. There was no clear correlation between levels of individual gangliosides and the genetic variant in Gaucher disease [9 samples of severe (neuronopathic), 4 samples of mild (non-neuronopathic) GBA variants, and 8 samples with low pathogenicity variants which have a higher risk for development of PD]. Most brain regions, i.e. occipital cortex, cingulate gyrus, and striatum, did not show a statistically significant elevation of GlcCer in PD-GBA. Only one region, the middle temporal gyrus, showed a small, but significant elevation in GlcCer concentration in PD-GBA. We conclude that changes in ganglioside, but not in GlcCer levels, may contribute to the association between PD and GBA mutations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

