The role of insulin and incretin-based drugs in biliary tract cancer: epidemiological and experimental evidence
- PMID: 35933633
- PMCID: PMC9357599
- DOI: 10.1007/s12672-022-00536-8
The role of insulin and incretin-based drugs in biliary tract cancer: epidemiological and experimental evidence
Abstract
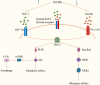
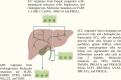
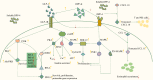
Insulin and incretin-based drugs are important antidiabetic agents with complex effects on cell growth and metabolism. Emerging evidence shows that insulin and incretin-based drugs are associated with altered risk of biliary tract cancer (BTC). Observational study reveals that insulin is associated with an increased risk of extrahepatic cholangiocarcinoma (ECC), but not intrahepatic cholangiocarcinoma (ICC) or gallbladder cancer (GBC). This type-specific effect can be partly explained by the cell of origin and heterogeneous genome landscape of the three subtypes of BTC. Similar to insulin, incretin-based drugs also exhibit very interesting contradictions and inconsistencies in response to different cancer phenotypes, including BTC. Both epidemiological and experimental evidence suggests that incretin-based drugs can be a promoter of some cancers and an inhibitor of others. It is now more apparent that this type of drugs has a broader range of physiological effects on the body, including regulation of endoplasmic reticulum stress, autophagy, metabolic reprogramming, and gene expression. In particular, dipeptidyl peptidase-4 inhibitors (DPP-4i) have a more complex effect on cancer due to the multi-functional nature of DPP-4. DPP-4 exerts both catalytic and non-enzymatic functions to regulate metabolic homeostasis, immune reaction, cell migration, and proliferation. In this review, we collate the epidemiological and experimental evidence regarding the effect of these two classes of drugs on BTC to provide valuable information.
Keywords: Antidiabetic; Biliary tract cancer; Cholangiocarcinoma; Incretin; Insulin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Insulin therapy and biliary tract cancer: insights from real-world data.Endocr Connect. 2022 Mar 14;11(3):e210546. doi: 10.1530/EC-21-0546. Endocr Connect. 2022. PMID: 35148280 Free PMC article.
-
Worldwide Incidence and Mortality of Biliary Tract Cancer.Gastro Hep Adv. 2022 Apr 15;1(4):618-626. doi: 10.1016/j.gastha.2022.04.007. eCollection 2022. Gastro Hep Adv. 2022. PMID: 39132071 Free PMC article.
-
Familial Risk of Biliary Tract Cancers: A Population-Based Study in Utah.Dig Dis Sci. 2016 Dec;61(12):3627-3632. doi: 10.1007/s10620-016-4310-3. Epub 2016 Sep 21. Dig Dis Sci. 2016. PMID: 27655103
-
Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes.Curr Med Res Opin. 2007 Apr;23(4):919-31. doi: 10.1185/030079906x162746. Curr Med Res Opin. 2007. PMID: 17407649 Review.
-
Promising Molecular Targets for the Targeted Therapy of Biliary Tract Cancers: An Overview.Onco Targets Ther. 2021 Feb 25;14:1341-1366. doi: 10.2147/OTT.S297643. eCollection 2021. Onco Targets Ther. 2021. PMID: 33658799 Free PMC article. Review.
Cited by
-
Liraglutide exhibits potential anti-tumor effects on the progression of intrahepatic cholangiocarcinoma, in vitro and in vivo.Sci Rep. 2024 Jun 14;14(1):13726. doi: 10.1038/s41598-024-64774-2. Sci Rep. 2024. PMID: 38877189 Free PMC article.
-
Involvement of interleukin-1β in high glucose-activated proliferation of cholangiocarcinoma.Transl Gastroenterol Hepatol. 2024 Jul 3;9:36. doi: 10.21037/tgh-24-8. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39091665 Free PMC article.
-
Association between incretin-based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: A large population-based matched cohort study.J Clin Transl Endocrinol. 2024 Sep 18;38:100370. doi: 10.1016/j.jcte.2024.100370. eCollection 2024 Dec. J Clin Transl Endocrinol. 2024. PMID: 39386155 Free PMC article.
-
Glucagon-like peptide 1 receptor agonist: A potential game changer for cholangiocarcinoma.World J Gastroenterol. 2024 Sep 14;30(34):3862-3867. doi: 10.3748/wjg.v30.i34.3862. World J Gastroenterol. 2024. PMID: 39350782 Free PMC article. Review.
References
-
- Valle JW, Lamarca A, Goyal L. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–62. doi: 10.1158/2159-8290.CD-17-0245. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
