PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion
- PMID: 35933642
- PMCID: PMC9804361
- DOI: 10.1002/jcp.30849
PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion
Abstract
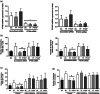
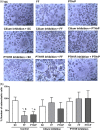
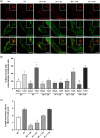
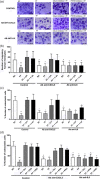
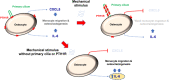
Osteocytes respond to mechanical forces controlling osteoblast and osteoclast function. Mechanical stimulation decreases osteocyte apoptosis and promotes bone formation. Primary cilia have been described as potential mechanosensors in bone cells. Certain osteogenic responses induced by fluid flow (FF) in vitro are decreased by primary cilia inhibition in MLO-Y4 osteocytes. The parathyroid hormone (PTH) receptor type 1 (PTH1R) modulates osteoblast, osteoclast, and osteocyte effects upon activation by PTH or PTH-related protein (PTHrP) in osteoblastic cells. Moreover, some actions of PTH1R seem to be triggered directly by mechanical stimulation. We hypothesize that PTH1R forms a signaling complex in the primary cilium that is essential for mechanotransduction in osteocytes and affects osteocyte-osteoclast communication. MLO-Y4 osteocytes were stimulated by FF or PTHrP (1-37). PTH1R and primary cilia signaling were abrogated using PTH1R or primary cilia specific siRNAs or inhibitors, respectively. Conditioned media obtained from mechanically- or PTHrP-stimulated MLO-Y4 cells inhibited the migration of preosteoclastic cells and osteoclast differentiation. Redistribution of PTH1R along the entire cilium was observed in mechanically stimulated MLO-Y4 osteocytic cells. Preincubation of MLO-Y4 cells with the Gli-1 antagonist, the adenylate cyclase inhibitor (SQ22536), or with the phospholipase C inhibitor (U73122), affected the migration of osteoclast precursors and osteoclastogenesis. Proteomic analysis and neutralizing experiments showed that FF and PTH1R activation control osteoclast function through the modulation of C-X-C Motif Chemokine Ligand 5 (CXCL5) and interleukin-6 (IL-6) secretion in osteocytes. These novel findings indicate that both primary cilium and PTH1R are necessary in osteocytes for proper communication with osteoclasts and show that mechanical stimulation inhibits osteoclast recruitment and differentiation through CXCL5, while PTH1R activation regulate these processes via IL-6.
Keywords: PTH1R; mechanotransduction; osteoclasts; osteocytes; primary cilia.
© 2022 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Figures


References
-
- Ardura, J. A. , Portal‐Núñez, S. , Alonso, V. , Bravo, B. , & Gortazar, A. R. (2019). Handling parathormone receptor type 1 in skeletal diseases: Realities and expectations of abaloparatide. Trends in Endocrinology and Metabolism, 30(10), 756–766. - PubMed
-
- Chen, X. , Macica, C. M. , Ng, K. W. , & Broadus, A. E. (2005). Stretch‐induced PTH—Related protein gene expression in osteoblasts. Journal of Bone and Mineral Research, 20(8), 1454–1461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

