Capillary electrophoresis Western blot using inkjet transfer to membrane
- PMID: 35933772
- PMCID: PMC12189877
- DOI: 10.1016/j.chroma.2022.463389
Capillary electrophoresis Western blot using inkjet transfer to membrane
Abstract
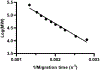
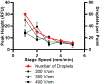
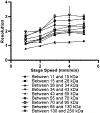
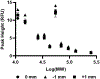
Traditional Western blots are commonly used to separate and assay proteins; however, they have limitations including a long, cumbersome process and large sample requirements. Here, we describe a system for Western blotting where capillary gel electrophoresis is used to separate sodium dodecyl sulfate-protein complexes. The capillary outlet is threaded into a piezoelectric inkjetting head that deposits the separated proteins in a quasi-continuous stream of <100 pL droplets onto a moving membrane. Through separations at 400 V/cm and protein capture on a membrane moving at 2 mm/min, we are able to detect actin with a limit of detection at 8 pM, or an estimated 5 fg injected. Separation and membrane capture of sample containing 10 proteins ranging in molecular weights from 11 - 250 kDa was achieved in 15 min. The system was demonstrated with Western blots for actin, β-tubulin, ERK1/2, and STAT3 in human A431 epidermoid carcinoma cell lysate.
Keywords: CE-SDS; Western blot; immunoassay; inkjet; separation.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Don T. Lamb reports financial support was provided by LI-COR Biosciences. Jon P. Anderson reports financial support was provided by LI-COR Biosciences. Michael D. Furtaw reports financial support was provided by LI-COR Biosciences. Michael D. Furtaw and Donald T. Lamb has patent Separation capillary inkjet dispensing with flat piezoelectric actuator pending to LI-COR, Inc. Michael D. Furtaw and Donald T. Lamb has patent Capillary electrophoresis inkjet dispensing issued to LI-COR, Inc.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

