The loss of pancreatic islet NADPH oxidase (NOX)2 improves islet transplantation
- PMID: 35933903
- PMCID: PMC9357848
- DOI: 10.1016/j.redox.2022.102419
The loss of pancreatic islet NADPH oxidase (NOX)2 improves islet transplantation
Abstract
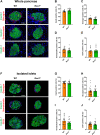
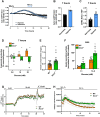
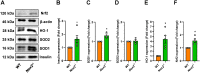
Islet transplantation is a promising treatment strategy for type 1 diabetes mellitus (T1DM) patients. However, oxidative stress-induced graft failure due to an insufficient revascularization is a major problem of this therapeutic approach. NADPH oxidase (NOX)2 is an important producer of reactive oxygen species (ROS) and several studies have already reported that this enzyme plays a crucial role in the endocrine function and viability of β-cells. Therefore, we hypothesized that targeting islet NOX2 improves the outcome of islet transplantation. To test this, we analyzed the cellular composition and viability of isolated wild-type (WT) and Nox2-/- islets by immunohistochemistry as well as different viability assays. Ex vivo, the effect of Nox2 deficiency on superoxide production, endocrine function and anti-oxidant protein expression was studied under hypoxic conditions. In vivo, we transplanted WT and Nox2-/- islets into mouse dorsal skinfold chambers and under the kidney capsule of diabetic mice to assess their revascularization and endocrine function, respectively. We found that the loss of NOX2 does not affect the cellular composition and viability of isolated islets. However, decreased superoxide production, higher glucose-stimulated insulin secretion as well as expression of nuclear factor erythroid 2-related factor (Nrf)2, heme oxygenase (HO)-1 and superoxide dismutase 1 (SOD1) was detected in hypoxic Nox2-/- islets when compared to WT islets. Moreover, we detected an early revascularization, a higher take rate and restoration of normoglycemia in diabetic mice transplanted with Nox2-/- islets. These findings indicate that the suppression of NOX2 activity represents a promising therapeutic strategy to improve engraftment and function of isolated islets.
Keywords: Diabetes; HO-1; Insulin secretion; Islet transplantation; NADPH oxidase; NOX2; Nrf2; ROS; Revascularization; β-cells.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Targeting Pancreatic Islet NLRP3 Improves Islet Graft Revascularization.Diabetes. 2022 Aug 1;71(8):1706-1720. doi: 10.2337/db21-0851. Diabetes. 2022. PMID: 35622000
-
NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion.Diabetes. 2012 Nov;61(11):2842-50. doi: 10.2337/db12-0009. Epub 2012 Aug 28. Diabetes. 2012. PMID: 22933115 Free PMC article.
-
BMAL1 modulates ROS generation and insulin secretion in pancreatic β-cells: An effect possibly mediated via NOX2.Mol Cell Endocrinol. 2022 Sep 15;555:111725. doi: 10.1016/j.mce.2022.111725. Epub 2022 Jul 20. Mol Cell Endocrinol. 2022. PMID: 35868425
-
NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice.Diabetes. 2010 Oct;59(10):2603-11. doi: 10.2337/db09-1562. Epub 2010 Jul 13. Diabetes. 2010. PMID: 20627937 Free PMC article.
-
The impact of oxidative stress on islet transplantation and monitoring the graft survival by non-invasive imaging.Curr Med Chem. 2013;20(9):1127-46. doi: 10.2174/0929867311320090003. Curr Med Chem. 2013. PMID: 23317098 Review.
Cited by
-
Heparan sulfate fine-tuned interleukin-1 (IL-1) signaling inhibits insulin secretion of grafted pancreatic islets.Sci Adv. 2025 Aug 8;11(32):eady8566. doi: 10.1126/sciadv.ady8566. Epub 2025 Aug 8. Sci Adv. 2025. PMID: 40779638 Free PMC article.
-
Redox Status as a Key Driver of Healthy Pancreatic Beta-Cells.Physiol Res. 2024 Aug 30;73(S1):S139-S152. doi: 10.33549/physiolres.935259. Epub 2024 Apr 22. Physiol Res. 2024. PMID: 38647167 Free PMC article. Review.
References
-
- Brissova M., et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–1325. - PubMed
-
- Emamaullee J.A., Shapiro A.M.J. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16(1):1–8. - PubMed
-
- Kim G., et al. Am J Transplant; 2020. Protective Effect of a Novel Clinical-Grade Small Molecule Necrosis Inhibitor against Oxidative Stress and Inflammation during Islet Transplantation. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

