The gut microbiome and Alzheimer's disease: Complex and bidirectional interactions
- PMID: 35934087
- PMCID: PMC9637435
- DOI: 10.1016/j.neubiorev.2022.104814
The gut microbiome and Alzheimer's disease: Complex and bidirectional interactions
Abstract
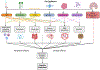
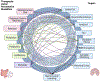
Structural and functional alterations to the gut microbiome, referred to as gut dysbiosis, have emerged as potential key mediators of neurodegeneration and Alzheimer disease (AD) pathogenesis through the "gut -brain" axis. Emerging data from animal and clinical studies support an important role for gut dysbiosis in mediating neuroinflammation, central and peripheral immune dysregulation, abnormal brain protein aggregation, and impaired intestinal and brain barrier permeability, leading to neuronal loss and cognitive impairment. Gut dysbiosis has also been shown to directly influence various mechanisms involved in neuronal growth and repair, synaptic plasticity, and memory and learning functions. Aging and lifestyle factors including diet, exercise, sleep, and stress influence AD risk through gut dysbiosis. Furthermore, AD is associated with characteristic gut microbial signatures which offer value as potential markers of disease severity and progression. Together, these findings suggest the presence of a complex bidirectional relationship between AD and the gut microbiome and highlight the utility of gut modulation strategies as potential preventative or therapeutic strategies in AD. We here review the current literature regarding the role of the gut-brain axis in AD pathogenesis and its potential role as a future therapeutic target in AD treatment and/or prevention.
Keywords: Age; Alzheimer disease; Gut microbiome; Lifestyle; Prevention.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures

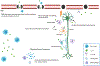
References
-
- Abraham D, Feher J, Scuderi GL, et al., 2019. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp. Gerontol 115, 122–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

