IFN-β1b induces OAS3 to inhibit EV71 via IFN-β1b/JAK/STAT1 pathway
- PMID: 35934228
- PMCID: PMC9583119
- DOI: 10.1016/j.virs.2022.07.013
IFN-β1b induces OAS3 to inhibit EV71 via IFN-β1b/JAK/STAT1 pathway
Abstract
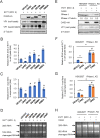
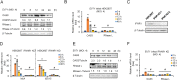
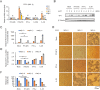
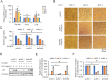
Enterovirus 71 (EV71) caused hand, foot and mouth disease (HFMD) is a serious threat to the health of young children. Although type I interferon (IFN-I) has been proven to control EV71 replication, the key downstream IFN-stimulated gene (ISG) remains to be clarified and investigated. Recently, we found that 2'-5'-oligoadenylate synthetases 3 (OAS3), as one of ISG of IFN-β1b, was antagonized by EV71 3C protein. Here, we confirm that OAS3 is the major determinant of IFN-β1b-mediated EV71 inhibition, which depends on the downstream constitutive RNase L activation. 2'-5'-oligoadenylate (2-5A) synthesis activity deficient mutations of OAS3 D816A, D818A, D888A, and K950A lost resistance to EV71 because they could not activate downstream RNase L. Further investigation proved that EV71 infection induced OAS3 but not RNase L expression by IFN pathway. Mechanically, EV71 or IFN-β1b-induced phosphorylation of STAT1, but not STAT3, initiated the transcription of OAS3 by directly binding to the OAS3 promoter. Our works elucidate the immune regulatory mechanism of the host OAS3/RNase L system against EV71 replication.
Keywords: 2′-5′-oligoadenylate synthetases 3 (OAS3); Enterovirus 71 (EV71); IFN-β1b; JAK/STAT; RNase L.
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest.
Figures

Similar articles
-
EV71 3C protease cleaves host anti-viral factor OAS3 and enhances virus replication.Virol Sin. 2022 Jun;37(3):418-426. doi: 10.1016/j.virs.2022.04.013. Epub 2022 May 3. Virol Sin. 2022. PMID: 35504537 Free PMC article.
-
Association of the OAS3 rs1859330 G/A genetic polymorphism with severity of enterovirus-71 infection in Chinese Han children.Arch Virol. 2017 Aug;162(8):2305-2313. doi: 10.1007/s00705-017-3381-6. Epub 2017 Apr 25. Arch Virol. 2017. PMID: 28444539
-
Oligoadenylate synthetase 3 S381R gene polymorphism is associated with severity of EV71 infection in Chinese children.J Clin Virol. 2018 Apr;101:29-33. doi: 10.1016/j.jcv.2018.01.015. Epub 2018 Jan 31. J Clin Virol. 2018. PMID: 29414184
-
OASs in Defense of Mycobacterial Infection: Angels or Demons?Curr Issues Mol Biol. 2021;40:221-230. doi: 10.21775/cimb.040.221. Epub 2020 Jul 1. Curr Issues Mol Biol. 2021. PMID: 32609093 Review.
-
Oligoadenylate and cyclic AMP: interrelation and mutual regulation.Prog Mol Subcell Biol. 1994;14:209-21. doi: 10.1007/978-3-642-78549-8_12. Prog Mol Subcell Biol. 1994. PMID: 7520328 Review.
Cited by
-
Research progress on pathogenic and therapeutic mechanisms of Enterovirus A71.Arch Virol. 2023 Sep 29;168(10):260. doi: 10.1007/s00705-023-05882-8. Arch Virol. 2023. PMID: 37773227 Review.
-
Cordycepin Inhibits Enterovirus A71 Replication and Protects Host Cell from Virus-Induced Cytotoxicity through Adenosine Action Pathway.Viruses. 2024 Feb 24;16(3):352. doi: 10.3390/v16030352. Viruses. 2024. PMID: 38543718 Free PMC article.
-
Adaptive Evolution of the OAS Gene Family Provides New Insights into the Antiviral Ability of Laurasiatherian Mammals.Animals (Basel). 2023 Jan 6;13(2):209. doi: 10.3390/ani13020209. Animals (Basel). 2023. PMID: 36670749 Free PMC article.
-
A CRISPR-Cas9 knockout screening identifies IRF2 as a key driver of OAS3/RNase L-mediated RNA decay during viral infection.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2412725121. doi: 10.1073/pnas.2412725121. Epub 2024 Oct 30. Proc Natl Acad Sci U S A. 2024. PMID: 39475651 Free PMC article.
-
Pterostilbene, an active constituent of blueberries, enhances innate immune activation and restricts enterovirus D68 infection.Front Immunol. 2023 Feb 9;14:1118933. doi: 10.3389/fimmu.2023.1118933. eCollection 2023. Front Immunol. 2023. PMID: 36845118 Free PMC article.
References
-
- Chebath J., Benech P., Revel M., Vigneron M. Constitutive expression of (2'-5') oligo a synthetase confers resistance to picornavirus infection. Nature. 1987;330:587–588. - PubMed
-
- Coccia E.M., Romeo G., Nissim A., Marziali G., Albertini R., Affabris E., Battistini A., Fiorucci G., Orsatti R., Rossi G.B., et al. A full-length murine 2-5a synthetase cdna transfected in nih-3t3 cells impairs emcv but not vsv replication. Virology. 1990;179:228–233. - PubMed
-
- Floyd-Smith G. (2'-5')an-dependent endoribonuclease: enzyme levels are regulated by ifn beta, ifn gamma, and cell culture conditions. J. Cell. Biochem. 1988;38:13–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

