Benzalkonium chloride, a common ophthalmic preservative, compromises rat corneal cold sensitive nerve activity
- PMID: 35934279
- PMCID: PMC12327410
- DOI: 10.1016/j.jtos.2022.07.012
Benzalkonium chloride, a common ophthalmic preservative, compromises rat corneal cold sensitive nerve activity
Abstract
Purpose: Corneal nerves comprise the densest sensory network in the body. Dysfunction of the corneal cold sensitive neurons (CSN) is implicated in ophthalmic disorders, including Dry Eye Disease, the most common ocular surface disorder. The preservative Benzalkonium chloride (BAK) and the mydriatic agent Phenylephrine hydrochloride (PHE) are considered to be inactive at the level of the CSNs. The purpose of this study is to test the impacts of continuous exposures to BAK or PHE at their clinically used concentrations on corneal nerve structure and function.
Methods: In vivo extracellular electrophysiology of the rat trigeminal ganglion was used to monitor CSN functional response to stimuli mimicking physiological states and stressors of the cornea. Corneal nerve structure was evaluated by immunostaining.
Results: Among the tested stimuli, cold probe receptive field stimulation and hyperosmolar stress were the most sensitive methods of detecting activity changes. CSN activity was attenuated after 30 min exposure to either PHE or BAK. After an hour-long washout period, BAK-treated neurons failed to recover activity while PHE-treated neurons showed signs of functional recovery. Intraepithelial nerve density was reduced and nerve fragmentation was increased in BAK-treated corneas, while PHE exposure left corneal nerves structurally intact.
Conclusions: Our study suggests that prolonged ocular instillations of BAK or PHE alter CSN activity through two different processes - irreversible neuronal damage in the case of BAK vs. reversible attenuation in the case of PHE.
Keywords: Benzalkonium chloride; Cold sensitive nerves; Cornea; In vivo electrophysiology; Nerve damage; Nerve function; Nerve structure; Sensation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts to disclose relevant to the production and publication of this work.
Figures

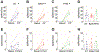
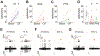

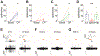


Similar articles
-
Hyaluronate Protects From Benzalkonium Chloride-Induced Ocular Surface Toxicity.Transl Vis Sci Technol. 2024 Oct 1;13(10):31. doi: 10.1167/tvst.13.10.31. Transl Vis Sci Technol. 2024. PMID: 39432403 Free PMC article.
-
Temporal evolution of the biological response to laser-induced refractive index change (LIRIC) in rabbit corneas.Exp Eye Res. 2021 Jun;207:108579. doi: 10.1016/j.exer.2021.108579. Epub 2021 Apr 20. Exp Eye Res. 2021. PMID: 33864783 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Astaxanthin ameliorates benzalkonium chloride-induced dry eye disease through suppressing inflammation and oxidative stress via Keap1-Nrf2/HO-1 signaling pathways.Animal Model Exp Med. 2025 Jun;8(6):1056-1079. doi: 10.1002/ame2.70000. Epub 2025 Mar 5. Animal Model Exp Med. 2025. PMID: 40045550 Free PMC article.
-
Preservative use in topical glaucoma medications.Ocul Surf. 2011 Jul;9(3):140-58. doi: 10.1016/s1542-0124(11)70024-6. Ocul Surf. 2011. PMID: 21791189
Cited by
-
Drug-free hyaluronic acid-microneedle with unexpected inhibition activity on benzalkonium chloride-induced corneal inflammation and stromal scarring.Mater Today Bio. 2025 Mar 29;32:101722. doi: 10.1016/j.mtbio.2025.101722. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40236813 Free PMC article.
-
Hyaluronate Protects From Benzalkonium Chloride-Induced Ocular Surface Toxicity.Transl Vis Sci Technol. 2024 Oct 1;13(10):31. doi: 10.1167/tvst.13.10.31. Transl Vis Sci Technol. 2024. PMID: 39432403 Free PMC article.
-
Development of a Temperature and pH Dual-Sensitive In-Situ Gel for Treating Allergic Conjunctivitis.AAPS PharmSciTech. 2024 Sep 25;25(7):223. doi: 10.1208/s12249-024-02931-6. AAPS PharmSciTech. 2024. PMID: 39322789
-
Deep Corneal Nerve Plexus Selective Damage in Persistent Neurotrophic Corneal Epithelial Defects Detected by In Vivo Multiphoton Confocal Microscopy.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):1. doi: 10.1167/iovs.66.4.1. Invest Ophthalmol Vis Sci. 2025. PMID: 40168155 Free PMC article.
-
A phase III, multicentre, randomised, investigator-masked, cross-over, comparative, non-inferiority trial evaluating the efficacy and tolerability of generic preservative-free Latanoprost (Polpharma S.A.) compared to Xalatan® (Pfizer) in patients with ocular hypertension or primary open-angle glaucoma.BMC Ophthalmol. 2024 Jul 29;24(1):313. doi: 10.1186/s12886-024-03579-3. BMC Ophthalmol. 2024. PMID: 39075412 Free PMC article. Clinical Trial.
References
-
- Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982;14(2):105–20. - PubMed
-
- Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investigative Ophthalmology & Visual Science 1989;30(3):461–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

