Time-restricted eating to improve cardiometabolic health: The New York Time-Restricted EATing randomized clinical trial - Protocol overview
- PMID: 35934281
- PMCID: PMC10031768
- DOI: 10.1016/j.cct.2022.106872
Time-restricted eating to improve cardiometabolic health: The New York Time-Restricted EATing randomized clinical trial - Protocol overview
Abstract
Re-aligning eating patterns with biological rhythm can reduce the burden of metabolic syndrome in older adults with overweight or obesity. Time-restricted eating (TRE) has been shown to result in weight loss and improved cardiometabolic health while being less challenging than counting calories. The New York Time-Restricted EATing study (NY-TREAT) is a two-arm, randomized clinical trial (RCT) that aims to examine the efficacy and sustainability of TRE (eating window ≤10 h/day) vs. a habitual prolonged eating window (HABIT, ≥14 h/day) in metabolically unhealthy midlife adults (50-75 years) with overweight or obesity and prediabetes or type 2 diabetes (T2D). Our primary hypothesis is that the TRE will result in greater weight loss compared to HABIT at 3 months. The efficacy of the TRE intervention on body weight, fat mass, energy expenditure, and glucose is tested at 3 months, and the sustainability of its effect is measured at 12 months, with ambulatory assessments of sleep and physical activity (ActiGraph), eating pattern (smartphone application), and interstitial glucose (continuous glucose monitoring). The RCT also includes state-of-the-art measurements of body fat (quantitative magnetic resonance), total energy expenditure (doubly-labelled water), insulin secretion, insulin resistance, and glucose tolerance. Adherence to self-monitoring and reduced eating window are monitored remotely in real-time. This RCT will provide further insight into the effects of TRE on cardiometabolic health in individuals with high metabolic risk. Sixty-two participants will be enrolled, and with estimated 30% attrition, 42 participants will return at 12 months. This protocol describes the design, interventions, methods, and expected outcomes. Clinical trial registration:NCT04465721 IRB: AAAS7791.
Keywords: Continuous glucose monitoring system; Diabetes; Doubly labelled water; Glucose; Meal timing; Prediabetes; Time-restricted eating.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CJP is a Sports Nutrition Consultant for Renaissance Periodization, LLC.
Figures

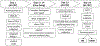
References
-
- CDC, National Diabetes Statistics Report, 2017 2017 The National Diabetes Statistics Report is a periodic publication of the Centersfor Disease Control and Prevention (CDC) that Provides Updated Statistics About Diabetes in the United States for a Scientific Audience. It Includesinformation on Prevalence and Incidence of Diabetes, Prediabetes, Risk Factors for Complications, Acute and Long-Term Complications, Deaths, and Costs. These Data can Help Focus Efforts to Prevent and Control Diabetes Across the United States. T, Available from, https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-stat..., 2017.
-
- Pi-Sunyer FX, Medical hazards of obesity, Ann. Intern. Med 119 (7 Pt 2) (1993) 655–660. - PubMed
-
- Liou T, Pi-Sunyer FX, Laferrère B, Physical disability and obesity, Nutr. Rev 63 (10) (2005) 321–331. - PubMed
-
- CDC, Promoting Preventative Services for Adults 50–64: Community and Clinical Partnerships, Available from, https://www.cdc.gov/aging/pdf/promoting-preventive-services.pdf, 2009.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

