Transitioning subcutaneous immunoglobulin 20% therapies in patients with primary and secondary immunodeficiencies: Canadian real-world study
- PMID: 35934726
- PMCID: PMC9358831
- DOI: 10.1186/s13223-022-00709-8
Transitioning subcutaneous immunoglobulin 20% therapies in patients with primary and secondary immunodeficiencies: Canadian real-world study
Erratum in
-
Correction: Transitioning subcutaneous immunoglobulin 20% therapies in patients with primary and secondary immunodeficiencies: Canadian real‑world study.Allergy Asthma Clin Immunol. 2023 Jun 23;19(1):54. doi: 10.1186/s13223-023-00805-3. Allergy Asthma Clin Immunol. 2023. PMID: 37353830 Free PMC article. No abstract available.
Abstract
Background: Real-world data on transitioning to Immune Globulin Subcutaneous (Human) 20% solution (Ig20Gly) are limited. This study aimed to assess infusion parameters and experience of patients with primary (PID) or secondary immunodeficiencies (SID) transitioning to Ig20Gly in clinical practice in Canada.
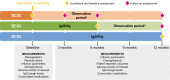
Methods: Patients with PID or SID who received subcutaneous immunoglobulin (SCIG) for ≥ 3 months before transitioning to Ig20Gly were eligible for this multicenter (n = 6), phase 4, non-interventional, prospective, single-arm study. Ig20Gly infusion parameters, dosing, and adverse events were collected from patient medical records at Ig20Gly initiation and 3, 6, and 12 months post-initiation. Patient satisfaction and quality of life were assessed 12 months post-initiation using validated questionnaires.
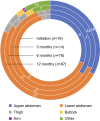
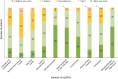
Results: The study included 125 patients (PID, n = 60; SID, n = 64; PID + SID, n = 1). Median volume per infusion was 30.0 ml at initiation, and 40.0 ml at 6 and 12 months post-initiation. Most patients administered Ig20Gly weekly and used two infusion sites (primarily abdomen). At each time point, median infusion duration was ≤ 1 h. At 12 months, 61% of infusions were administered via a pump and 39% manually. Headache and infusion-site reactions were the most reported adverse events of interest. Patients expressed overall satisfaction with Ig20Gly at 12 months post-initiation, with all respondents indicating they would like to continue Ig20Gly.
Conclusions: This study provides a detailed description of Ig20Gly infusion parameters, tolerability, and quality of life in clinical practice among patients with PID or SID switching to Ig20Gly from another SCIG and confirms the feasibility of infusing Ig20Gly via pump or manual administration. Trial registration NCT03716700, Registered 31 August 2018, https://clinicaltrials.gov/ct2/show/NCT03716700.
Keywords: Ig20Gly; Immunoglobulin replacement therapy; Primary immunodeficiency diseases; Real-world; Secondary immunodeficiency diseases; Subcutaneous immunoglobulin.
© 2022. The Author(s).
Conflict of interest statement
PKK has received personal fees from Novartis, Aralez Pharmaceuticals, Pediapharm, AstraZeneca, Shire, CSL Behring, Kaleo, Merck, GlaxoSmithKline, Mylan, ALK, Sanofi, and Stallergenes and grants from AstraZeneca, CSL Behring, Genentech, and Shire. JCo has received research funding not related to this study from CSL Behring, Grifols, and Octapharma. She has received speaker fees from Octapharma, GSK, EMD Serono, and Alexion Pharma. She has received educational funding from Takeda. AK has received personal fees from Novartis, Aralez Pharmaceuticals, Pediapharm, Takeda, CSL Behring, and Mylan. He has received grants from Genentech and Takeda. HK has been on speakers’ bureaus and/or advisory boards for ALK, AstraZeneca, Aralez, CSL Behring, Kaleo, Merck, Mylan, Novartis, Pediapharm, Sanofi, and Shire. He has received research funding from AstraZeneca, Shire, and Novartis. GL has participated in research with Takeda. She has received speaker fees from Takeda, CSL Behring, Novartis, AstraZeneca, and Mylan. She has participated in advisory boards for Takeda, CSL Behring, Octapharma, Sanofi, GSK, and AstraZeneca; and was sponsored to attend international meetings with Shire/Takeda, AstraZeneca, and GSK. JKL has received speaker fees from ALK, Paladin, Novartis, AstraZeneca, Sanofi, Astellas, GSK, Takeda, Merck, CSL Behring, Omega, Sanofi Genzyme, Mylan, Meda, Pediapharm, Aralez, Paladin, Pfizer, and Stallergenes. He has received grants and research funding from Immunodeficiency Canada, AstraZeneca, ALK, Aralez, Paladin, Novartis, Sanofi, CSL Behring, GSK, Pediapharm, Stallergenes, Roche-Genentech, PPD, Shire, Takeda, Trudell Medical, Bausch and Lomb, and Regeneron. He has participated in advisory boards for GSK, Novartis, AstraZeneca, Merck, CSL Behring, Teva, Sanofi Genzyme, Mylan, ALK, Meda, Pediapharm, Paladin, Regeneron, AbbVie, and Genentech. He has been a consultant for GSK, AstraZeneca, Novartis, Regeneron, and Sanofi Genzyme. JCh and MP are employees of Takeda Development Center Americas, Inc. and are Takeda shareholders. AG is an employee of Takeda Pharmaceuticals International AG and a Takeda shareholder.
Figures
Similar articles
-
Effectiveness, Safety, and Treatment Satisfaction of 20% Subcutaneous Immunoglobulin Replacement Therapy in Pediatric Patients with Primary and Secondary Immunodeficiencies.Turk Arch Pediatr. 2025 Mar 3;60(2):217-225. doi: 10.5152/TurkArchPediatr.2025.24305. Turk Arch Pediatr. 2025. PMID: 40094369 Free PMC article.
-
Infusion parameters, safety, and practical guidance for the manual administration of subcutaneous immunoglobulin 20% (Ig20Gly).Allergy Asthma Clin Immunol. 2024 Oct 4;20(1):52. doi: 10.1186/s13223-024-00914-7. Allergy Asthma Clin Immunol. 2024. PMID: 39367472 Free PMC article. Review.
-
Real-World Use, Safety, and Patient Experience of 20% Subcutaneous Immunoglobulin for Primary Immunodeficiency Diseases.Adv Ther. 2023 Dec;40(12):5168-5187. doi: 10.1007/s12325-023-02649-0. Epub 2023 Sep 26. Adv Ther. 2023. PMID: 37751025 Free PMC article.
-
Infusion parameters of 20% subcutaneous immunoglobulin for primary immunodeficiency diseases among patient support program participants.Ann Allergy Asthma Immunol. 2021 Nov;127(5):568-574.e1. doi: 10.1016/j.anai.2021.06.023. Epub 2021 Jul 2. Ann Allergy Asthma Immunol. 2021. PMID: 34224864
-
Long-Term Efficacy and Safety of Hizentra® in Patients with Primary Immunodeficiency in Japan, Europe, and the United States: a Review of 7 Phase 3 Trials.J Clin Immunol. 2018 Nov;38(8):864-875. doi: 10.1007/s10875-018-0560-5. Epub 2018 Nov 10. J Clin Immunol. 2018. PMID: 30415311 Free PMC article. Review.
Cited by
-
A Phase 1 Open-Label Study to Assess the Tolerability, Safety, and Immunogenicity of Hyaluronidase-Facilitated Subcutaneous Immunoglobulin 20% in Healthy Adults.J Clin Immunol. 2023 Dec 22;44(1):28. doi: 10.1007/s10875-023-01632-2. J Clin Immunol. 2023. PMID: 38129731 Free PMC article. Clinical Trial.
-
Effectiveness, Safety, and Treatment Satisfaction of 20% Subcutaneous Immunoglobulin Replacement Therapy in Pediatric Patients with Primary and Secondary Immunodeficiencies.Turk Arch Pediatr. 2025 Mar 3;60(2):217-225. doi: 10.5152/TurkArchPediatr.2025.24305. Turk Arch Pediatr. 2025. PMID: 40094369 Free PMC article.
-
Patient-reported outcomes with subcutaneous immunoglobulin in secondary immunodeficiency.Front Immunol. 2025 Mar 20;16:1528414. doi: 10.3389/fimmu.2025.1528414. eCollection 2025. Front Immunol. 2025. PMID: 40181959 Free PMC article. Review.
-
Infusion parameters, safety, and practical guidance for the manual administration of subcutaneous immunoglobulin 20% (Ig20Gly).Allergy Asthma Clin Immunol. 2024 Oct 4;20(1):52. doi: 10.1186/s13223-024-00914-7. Allergy Asthma Clin Immunol. 2024. PMID: 39367472 Free PMC article. Review.
-
Immunoglobulin utilization in Canada: a comparative analysis of provincial guidelines and a scoping review of the literature.Allergy Asthma Clin Immunol. 2023 Sep 16;19(1):85. doi: 10.1186/s13223-023-00841-z. Allergy Asthma Clin Immunol. 2023. PMID: 37717038 Free PMC article.
References
-
- Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(1186–205):e1–78. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

