Cost-Effectiveness Analysis of Stockholm 3 Testing Compared to PSA as the Primary Blood Test in the Prostate Cancer Diagnostic Pathway: A Decision Tree Approach
- PMID: 35934771
- PMCID: PMC9596577
- DOI: 10.1007/s40258-022-00741-0
Cost-Effectiveness Analysis of Stockholm 3 Testing Compared to PSA as the Primary Blood Test in the Prostate Cancer Diagnostic Pathway: A Decision Tree Approach
Abstract
Objective: This study evaluated the cost effectiveness of using Stockholm 3 (STHLM3) testing compared to the prostate-specific antigen (PSA) test in the diagnostic pathway for prostate cancer.
Methods: We created a decision tree model for PSA (current standard) and STHLM3 (new alternative). Cost effectiveness was evaluated in a hypothetical cohort of male individuals aged 50-69 years. The study applied a Danish hospital perspective with a time frame restricted to the prostate cancer diagnostic pathway, beginning with the initial PSA/STHLM3 test, and ending with biopsy and histopathological diagnosis. Estimated values from the decision-analytical model were used to calculate the incremental cost-effectiveness ratio. Deterministic and probabilistic sensitivity analyses were conducted to test the robustness of the base-case analysis.
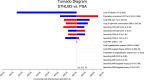
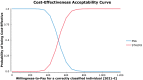
Results: The model-based analysis revealed that STHLM3 testing was more effective than the PSA, but also more costly, with an incremental cost-effectiveness ratio of €511.7 (95% credible interval, 359.9-674.3) for each additional correctly classified individual. In the deterministic sensitivity analysis, variations in the cost of STHLM3 had the greatest influence on the incremental cost-effectiveness ratio. In the probabilistic sensitivity analysis, all iterations were positioned in the north-east quadrant of the incremental cost-effectiveness scatterplot. At a willingness to pay of €700 for an additional correctly classified individual, STHLM3 had a 100% probability of being cost effective.
Conclusions: Compared to the PSA test as the initial testing modality in the prostate cancer diagnostic workup, STHLM3 testing showed improved incremental effectiveness, however, at additional costs. The results were sensitive to the cost of the STHLM3 test; therefore, a lower cost of the STHLM3 test would improve its cost effectiveness compared with PSA tests.
© 2022. The Author(s).
Figures


Similar articles
-
Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study.Lancet Oncol. 2015 Dec;16(16):1667-76. doi: 10.1016/S1470-2045(15)00361-7. Epub 2015 Nov 10. Lancet Oncol. 2015. PMID: 26563502
-
Prostate cancer screening in men aged 50 to 69 years (STHLM3): A prospective population-based diagnostic study. Grönberg H, Adolfsson J, Aly M, Nordström T, Wiklund P, Brandberg Y, Thompson J, Wiklund F, Lindberg J, Clements M, Egevad L, Eklund M.Lancet Oncol. 2015 Dec;16(16):1667-76. [Epub 2015 Nov 10]. doi: 10.1016/S1470-2045(15)00361-7.Urol Oncol. 2017 Mar;35(3):120. doi: 10.1016/j.urolonc.2016.03.013. Urol Oncol. 2017. PMID: 28215847
-
Cost-effectiveness of Prostate Cancer Screening Using Magnetic Resonance Imaging or Standard Biopsy Based on the STHLM3-MRI Study.JAMA Oncol. 2022 Nov 10;9(1):88-94. doi: 10.1001/jamaoncol.2022.5252. Online ahead of print. JAMA Oncol. 2022. PMID: 36355382 Free PMC article.
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
-
Laparoscopic versus open subtotal gastrectomy for gastric adenocarcinoma: cost-effectiveness analysis.BJS Open. 2020 Oct;4(5):830-839. doi: 10.1002/bjs5.50327. Epub 2020 Aug 6. BJS Open. 2020. PMID: 32762036 Free PMC article. Review.
Cited by
-
Advancements in Biomarkers of Prostate Cancer: A Review.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241290029. doi: 10.1177/15330338241290029. Technol Cancer Res Treat. 2024. PMID: 39440372 Free PMC article. Review.
-
Molecular diagnostics of prostate cancer: impact of molecular tests.Asian J Androl. 2024 Nov 1;26(6):562-566. doi: 10.4103/aja202411. Epub 2024 May 10. Asian J Androl. 2024. PMID: 38738960 Free PMC article. Review.
-
Cancer screening in patients with acromegaly: a plea for a personalized approach and international registries.Rev Endocr Metab Disord. 2025 Aug;26(4):525-538. doi: 10.1007/s11154-025-09957-6. Epub 2025 Mar 15. Rev Endocr Metab Disord. 2025. PMID: 40088375 Free PMC article. Review.
-
Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer.J Pers Med. 2024 Jan 23;14(2):130. doi: 10.3390/jpm14020130. J Pers Med. 2024. PMID: 38392564 Free PMC article.
-
Systematic Review on the Cost Effectiveness of Prostate Cancer Screening in Europe.Eur Urol. 2024 Nov;86(5):400-408. doi: 10.1016/j.eururo.2024.04.036. Epub 2024 May 23. Eur Urol. 2024. PMID: 38789306 Free PMC article.
References
-
- NORCAN. Prediction: tables. 2021. https://nordcan.iarc.fr/en/dataviz/predictions_tables?cancers=240&map_vi.... Accessed 23 Nov 2021.
-
- Gronberg H, Adolfsson J, Aly M, Nordstrom T, Wiklund P, Brandberg Y, et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16(16):1667–1676. - PubMed
-
- Gronberg H, Eklund M, Picker W, Aly M, Jaderling F, Adolfsson J, et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol. 2018;74(6):722–728. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

