Targeting SAMHD1 with hydroxyurea in first-line cytarabine-based therapy of newly diagnosed acute myeloid leukaemia: Results from the HEAT-AML trial
- PMID: 35934913
- PMCID: PMC9643609
- DOI: 10.1111/joim.13553
Targeting SAMHD1 with hydroxyurea in first-line cytarabine-based therapy of newly diagnosed acute myeloid leukaemia: Results from the HEAT-AML trial
Abstract
Background: Treatment of newly diagnosed acute myeloid leukaemia (AML) is based on combination chemotherapy with cytarabine (ara-C) and anthracyclines. Five-year overall survival is below 30%, which has partly been attributed to cytarabine resistance. Preclinical data suggest that the addition of hydroxyurea potentiates cytarabine efficacy by increasing ara-C triphosphate (ara-CTP) levels through targeted inhibition of SAMHD1.
Objectives: In this phase 1 trial, we evaluated the feasibility, safety and efficacy of the addition of hydroxyurea to standard chemotherapy with cytarabine/daunorubicin in newly diagnosed AML patients.
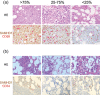
Methods: Nine patients were enrolled and received at least two courses of ara-C (1 g/m2 /2 h b.i.d. d1-5, i.e., a total of 10 g/m2 per course), hydroxyurea (1-2 g d1-5) and daunorubicin (60 mg/m2 d1-3). The primary endpoint was safety; secondary endpoints were complete remission rate and measurable residual disease (MRD). Additionally, pharmacokinetic studies of ara-CTP and ex vivo drug sensitivity assays were performed.
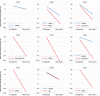
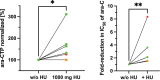
Results: The most common grade 3-4 toxicity was febrile neutropenia (100%). No unexpected toxicities were observed. Pharmacokinetic analyses showed a significant increase in median ara-CTP levels (1.5-fold; p = 0.04) in patients receiving doses of 1 g hydroxyurea. Ex vivo, diagnostic leukaemic bone marrow blasts from study patients were significantly sensitised to ara-C by a median factor of 2.1 (p = 0.0047). All nine patients (100%) achieved complete remission, and all eight (100%) with validated MRD measurements (flow cytometry or real-time quantitative polymerase chain reaction [RT-qPCR]) had an MRD level <0.1% after two cycles of chemotherapy. Treatment was well-tolerated, and median time to neutrophil recovery >1.0 × 109 /L and to platelet recovery >50 × 109 /L after the start of cycle 1 was 19 days and 22 days, respectively. Six of nine patients underwent allogeneic haematopoietic stem-cell transplantation (allo-HSCT). With a median follow-up of 18.0 (range 14.9-20.5) months, one patient with adverse risk not fit for HSCT experienced a relapse after 11.9 months but is now in second complete remission.
Conclusion: Targeted inhibition of SAMHD1 by the addition of hydroxyurea to conventional AML therapy is safe and appears efficacious within the limitations of the small phase 1 patient cohort. These results need to be corroborated in a larger study.
Keywords: SAMHD1; acute myeloid leukaemia; cytarabine; hydroxyurea; precision medicine; targeted therapy.
© 2022 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
T. S. is employed by Heidelberg ImmunoTherapeutics, not relevant to this work. J. I. H. is a consultant for SOBI, not relevant to this work. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Targeting SAMHD1 with the Vpx protein to improve cytarabine therapy for hematological malignancies.Nat Med. 2017 Feb;23(2):256-263. doi: 10.1038/nm.4265. Epub 2017 Jan 9. Nat Med. 2017. PMID: 28067901
-
SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia.Nat Med. 2017 Feb;23(2):250-255. doi: 10.1038/nm.4255. Epub 2016 Dec 19. Nat Med. 2017. PMID: 27991919
-
Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: a multicentre, single-arm, phase 2 trial.Lancet Haematol. 2022 Jun;9(6):e415-e424. doi: 10.1016/S2352-3026(22)00106-5. Epub 2022 May 2. Lancet Haematol. 2022. PMID: 35512726 Clinical Trial.
-
Midostaurin in Combination With Standard Chemotherapy for Treatment of Newly Diagnosed FMS-Like Tyrosine Kinase 3 (FLT3) Mutation-Positive Acute Myeloid Leukemia.Ann Pharmacother. 2018 Apr;52(4):364-369. doi: 10.1177/1060028017747900. Epub 2017 Dec 12. Ann Pharmacother. 2018. PMID: 29231051 Review.
-
EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes.Oncologist. 2020 Sep;25(9):e1414-e1420. doi: 10.1634/theoncologist.2019-0785. Epub 2020 Apr 13. Oncologist. 2020. PMID: 32282100 Free PMC article. Review.
Cited by
-
SAMHD1 shapes deoxynucleotide triphosphate homeostasis by interconnecting the depletion and biosynthesis of different dNTPs.Nat Commun. 2025 Jan 18;16(1):793. doi: 10.1038/s41467-025-56208-y. Nat Commun. 2025. PMID: 39824836 Free PMC article.
-
Hypomethylating agents plus modified priming regimens compared with venetoclax-based regimens based on molecular characteristics for newly diagnosed patients with acute myeloid leukemia: a multi-center cohort study.Ann Hematol. 2023 Dec;102(12):3369-3381. doi: 10.1007/s00277-023-05452-7. Epub 2023 Sep 18. Ann Hematol. 2023. PMID: 37723307
-
Activation of STING by SAMHD1 Deficiency Promotes PANoptosis and Enhances Efficacy of PD-L1 Blockade in Diffuse Large B-cell Lymphoma.Int J Biol Sci. 2023 Aug 28;19(14):4627-4643. doi: 10.7150/ijbs.85236. eCollection 2023. Int J Biol Sci. 2023. PMID: 37781035 Free PMC article.
-
AHR signaling pathway mediates mitochondrial oxidative phosphorylation which leads to cytarabine resistance.Acta Biochim Biophys Sin (Shanghai). 2024 Apr 25;56(4):597-606. doi: 10.3724/abbs.2024022. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38404179 Free PMC article.
-
Identification and evaluation of small-molecule inhibitors against the dNTPase SAMHD1 via a comprehensive screening funnel.iScience. 2024 Jan 13;27(2):108907. doi: 10.1016/j.isci.2024.108907. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318365 Free PMC article.
References
-
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–87. - PubMed
-
- Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish Acute Leukemia Registry. Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S54–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

