A simple and versatile fluorochrome-based procedure for imaging of lipids in arbuscule-containing cells
- PMID: 35934996
- PMCID: PMC9804681
- DOI: 10.1111/tpj.15934
A simple and versatile fluorochrome-based procedure for imaging of lipids in arbuscule-containing cells
Abstract
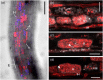
The arbuscular mycorrhizal (AM) symbiosis is characterized by the reciprocal exchange of nutrients. AM fungi are oleaginous microorganisms that obtain essential fatty acids from host plants. A lipid biosynthesis and delivery pathway has been proposed to operate in inner root cortex cells hosting arbuscules, a cell type challenging to access microscopically. Despite the central role lipids play in the association, lipid distribution patterns during arbuscule development are currently unknown. We developed a simple co-staining method employing fluorophore-conjugated Wheat Germ Agglutinin (WGA) and a lipophilic blue fluorochrome, Ac-201, for the simultaneous imaging of arbuscules and lipids distributed within arbuscule-containing cells in high resolution. We observed lipid distribution patterns in wild-type root infection zones in a variety of plant species. In addition, we applied this methodology to mutants of the Lotus japonicus GRAS transcription factor RAM1 and the Oryza sativa half-size ABC transporter STR1, both proposed to be impaired in the symbiotic lipid biosynthesis-delivery pathway. We found that lipids accumulated in cortical cells hosting stunted arbuscules in Ljram1 and Osstr1, and observed lipids in the arbuscule body of Osstr1, suggesting that in the corresponding plant species, RAM1 and STR1 may not be essential for symbiotic lipid biosynthesis and transfer from arbuscule-containing cells, respectively. The versatility of this methodology has the potential to help elucidate key questions on the complex lipid dynamics fostering AM symbioses.
Keywords: arbuscular mycorrhizal symbiosis; arbuscule-containing cells; cell biology; confocal microscopy; lipids; technical advance.
© 2022 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


Similar articles
-
The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice.Plant J. 2012 Mar;69(5):906-20. doi: 10.1111/j.1365-313X.2011.04842.x. Epub 2011 Dec 15. Plant J. 2012. PMID: 22077667
-
Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis.Plant Cell. 2010 May;22(5):1483-97. doi: 10.1105/tpc.110.074955. Epub 2010 May 7. Plant Cell. 2010. PMID: 20453115 Free PMC article.
-
The GRAS protein RAM1 interacts with WRI transcription factors to regulate plant genes required for arbuscule development and function.Proc Natl Acad Sci U S A. 2025 May 27;122(21):e2427021122. doi: 10.1073/pnas.2427021122. Epub 2025 May 19. Proc Natl Acad Sci U S A. 2025. PMID: 40388617 Free PMC article.
-
Through the doors of perception to function in arbuscular mycorrhizal symbioses.New Phytol. 2014 Dec;204(4):833-40. doi: 10.1111/nph.12862. Epub 2014 May 28. New Phytol. 2014. PMID: 25414918 Review.
-
Plant carbon nourishment of arbuscular mycorrhizal fungi.Curr Opin Plant Biol. 2017 Oct;39:50-56. doi: 10.1016/j.pbi.2017.05.008. Epub 2017 Jun 8. Curr Opin Plant Biol. 2017. PMID: 28601651 Review.
Cited by
-
Exogenous myristate promotes the colonization of arbuscular mycorrhizal fungi in tomato.Front Plant Sci. 2023 Nov 2;14:1250684. doi: 10.3389/fpls.2023.1250684. eCollection 2023. Front Plant Sci. 2023. PMID: 38023845 Free PMC article.
-
The AMSlide for noninvasive time-lapse imaging of arbuscular mycorrhizal symbiosis.J Microsc. 2025 Mar;297(3):289-303. doi: 10.1111/jmi.13313. Epub 2024 May 15. J Microsc. 2025. PMID: 38747391 Free PMC article.
References
-
- Becard, G. & Fortin, J.A. (1988) Early events of vesicular‐arbuscular mycorrhiza formation on Ri T‐DNA transformed roots. The New Phytologist, 108, 211–218. - PubMed
-
- Bonfante‐Fasolo, P. (1984) Anatomy and morphology of VA mycorrhizae. In: Powell, C.L. & Bagyaraj, D.J. (Eds.) VA mycorrhizae. Boca Raton: CRC Press, pp. 5–33.
-
- Brands, M. , Wewer, V. , Keymer, A. , Gutjahr, C. & Dormann, P. (2018) The Lotus japonicus acyl‐acyl carrier protein thioesterase FatM is required for mycorrhiza formation and lipid accumulation of Rhizophagus irregularis . The Plant Journal, 95, 219–232. - PubMed
-
- Bravo, A. , Brands, M. , Wewer, V. , Dormann, P. & Harrison, M.J. (2017) Arbuscular mycorrhiza‐specific enzymes FatM and RAM2 fine‐tune lipid biosynthesis to promote development of arbuscular mycorrhiza. The New Phytologist, 214, 1631–1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

