Single-atom logic for heterocycle editing
- PMID: 35935106
- PMCID: PMC9355079
- DOI: 10.1038/s44160-022-00052-1
Single-atom logic for heterocycle editing
Abstract
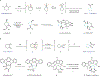
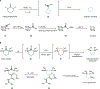
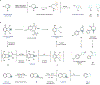
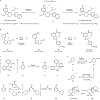
Medicinal chemistry continues to be impacted by new synthetic methods. Particularly sought after, especially at the drug discovery stage, is the ability to enact the desired chemical transformations in a concise and chemospecific fashion. To this end, the field of organic synthesis has become captivated by the idea of 'molecular editing'-to rapidly build onto, change or prune molecules one atom at a time using transformations that are mild and selective enough to be employed at the late stages of a synthetic sequence. In this Review, the definition and categorization of a particularly promising subclass of molecular editing reactions, termed 'single-atom skeletal editing', are proposed. Although skeletal editing applies to both cyclic and acyclic compounds, this Review focuses on heterocycles, both for their centrality in medicinal chemistry and for the definitional clarity afforded by a focus on ring systems. A classification system is presented by highlighting methods (both historically important examples and recent advances) that achieve such transformations, with the goal to spark interest and inspire further development in this growing field.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures






References
-
- Blakemore DC et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem 10, 383–394 (2018). - PubMed
-
- Campos KR et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019). - PubMed
-
- Hann MM & Keserü GM Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov 11, 355–365 (2012). - PubMed
-
- Vitaku E, Smith DT & Njardarson JT Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem 57, 10257–10274 (2014). - PubMed
-
- Taylor RD, MacCoss M & Lawson ADG Rings in Drugs. J. Med. Chem 57, 5845–5859 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
