Nanozyme-Triggered Cascade Reactions from Cup-Shaped Nanomotors Promote Active Cellular Targeting
- PMID: 35935135
- PMCID: PMC9275069
- DOI: 10.34133/2022/9831012
Nanozyme-Triggered Cascade Reactions from Cup-Shaped Nanomotors Promote Active Cellular Targeting
Abstract
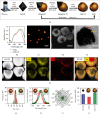
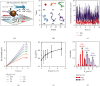
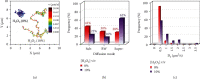
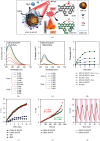
Self-propelled nanomotors have shown enormous potential in biomedical applications. Herein, we report on a nanozyme-powered cup-shaped nanomotor for active cellular targeting and synergistic photodynamic/thermal therapy under near-infrared (NIR) laser irradiation. The nanomotor is constructed by the asymmetric decoration of platinum nanoparticles (PtNPs) at the bottom of gold nanocups (GNCs). PtNPs with robust peroxidase- (POD-) like activity are employed not only as propelling elements for nanomotors but also as continuous O2 generators to promote photodynamic therapy via catalyzing endogenous H2O2 decomposition. Owing to the Janus structure, asymmetric propulsion force is generated to trigger the short-ranged directional diffusion, facilitating broader diffusion areas and more efficient cellular searching and uptake. This cascade strategy combines key capabilities, i.e., endogenous substrate-based self-propulsion, active cellular targeting, and enhanced dual-modal therapy, in one multifunctional nanomotor, which is crucial in advancing self-propelled nanomotors towards eventual therapeutic agents.
Copyright © 2022 Xin Wang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures


Similar articles
-
Multi-phoretic nanomotor with consistent motion direction for enhanced cancer therapy.Acta Biomater. 2025 Jan 1;191:352-368. doi: 10.1016/j.actbio.2024.11.037. Epub 2024 Nov 24. Acta Biomater. 2025. PMID: 39586348
-
Dual source-powered multifunctional Pt/FePc@Mn-MOF spindle-like Janus nanomotors for active CT imaging-guided synergistic photothermal/chemodynamic therapy.J Colloid Interface Sci. 2024 Mar;657:799-810. doi: 10.1016/j.jcis.2023.12.018. Epub 2023 Dec 6. J Colloid Interface Sci. 2024. PMID: 38081114
-
Photothermal interference urease-powered polydopamine nanomotor for enhanced propulsion and synergistic therapy.Colloids Surf B Biointerfaces. 2022 Apr;212:112353. doi: 10.1016/j.colsurfb.2022.112353. Epub 2022 Jan 22. Colloids Surf B Biointerfaces. 2022. PMID: 35085936
-
Janus micro/nanomotors for enhanced disease treatment through their deep penetration capability.Acta Biomater. 2025 Apr;196:50-77. doi: 10.1016/j.actbio.2025.02.055. Epub 2025 Feb 25. Acta Biomater. 2025. PMID: 40015356 Review.
-
Unfolding the future: Self-controlled catalytic nanomotor in healthcare system.Mater Sci Eng C Mater Biol Appl. 2020 Dec;117:111330. doi: 10.1016/j.msec.2020.111330. Epub 2020 Aug 7. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32919683 Review.
Cited by
-
Novel platinum therapeutics induce rapid cancer cell death through triggering intracellular ROS storm.Biomaterials. 2025 Mar;314:122835. doi: 10.1016/j.biomaterials.2024.122835. Epub 2024 Sep 11. Biomaterials. 2025. PMID: 39276409
-
Nanomaterial-assisted theranosis of bone diseases.Bioact Mater. 2022 Dec 26;24:263-312. doi: 10.1016/j.bioactmat.2022.12.014. eCollection 2023 Jun. Bioact Mater. 2022. PMID: 36632509 Free PMC article. Review.
-
Terahertz Wave Alleviates Comorbidity Anxiety in Pain by Reducing the Binding Capacity of Nanostructured Glutamate Molecules to GluA2.Research (Wash D C). 2024 Dec 11;7:0535. doi: 10.34133/research.0535. eCollection 2024. Research (Wash D C). 2024. PMID: 39664293 Free PMC article.
-
Thermal and Magnetic Dual-Responsive Catheter-Assisted Shape Memory Microrobots for Multistage Vascular Embolization.Research (Wash D C). 2024 Mar 26;7:0339. doi: 10.34133/research.0339. eCollection 2024. Research (Wash D C). 2024. PMID: 38550780 Free PMC article.
-
Engineering Cup-Shaped Nanomotors for Promoting Cell Internalization and Synergistic Tumor Therapy.Research (Wash D C). 2025 Apr 22;8:0623. doi: 10.34133/research.0623. eCollection 2025. Research (Wash D C). 2025. PMID: 40264654 Free PMC article.
References
-
- Yang J., Yang Y.-W. Metal-organic framework-based cancer theranostic nanoplatforms. View . 2020;1(2):p. e20. doi: 10.1002/viw2.20. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

