Osteocytes Serve as a Reservoir for Intracellular Persisting Staphylococcus aureus Due to the Lack of Defense Mechanisms
- PMID: 35935196
- PMCID: PMC9355688
- DOI: 10.3389/fmicb.2022.937466
Osteocytes Serve as a Reservoir for Intracellular Persisting Staphylococcus aureus Due to the Lack of Defense Mechanisms
Abstract
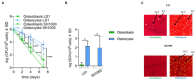
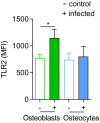
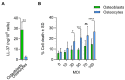
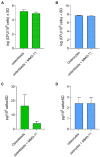
Chronic staphylococcal osteomyelitis can persist for long time periods causing bone destruction. The ability of Staphylococcus aureus to develop chronic infections is linked to its capacity to invade and replicate within osteoblasts and osteocytes and to switch to a dormant phenotype called small colony variants. Recently, osteocytes were described as a main reservoir for this pathogen in bone tissue. However, the mechanisms involved in the persistence of S. aureus within these cells are still unknown. Here, we investigated the interaction between S. aureus and osteoblasts or osteocytes during infection. While osteoblasts are able to induce a strong antimicrobial response and eliminate intracellular S. aureus, osteocytes trigger signals to recruit immune cells and enhance inflammation but fail an efficient antimicrobial activity to clear the bacterial infection. Moreover, we found that extracellular signals from osteocytes enhance intracellular bacterial clearance by osteoblasts. Even though both cell types express Toll-like receptor (TLR) 2, the main TLR responsible for S. aureus detection, only osteoblasts were able to increase TLR2 expression after infection. Additionally, proteomic analysis indicates that reduced intracellular bacterial killing activity in osteocytes is related to low antimicrobial peptide expression. Nevertheless, high levels of lipid mediators and cytokines were secreted by osteocytes, suggesting that they can contribute to inflammation. Taken together, our results demonstrate that osteocytes contribute to severe inflammation observed in osteomyelitis and represent the main niche for S. aureus persistence due to their poor capacity for intracellular antimicrobial response.
Keywords: S. aureus; chronic osteomyelitis; osteoblasts; osteocytes; persistence.
Copyright © 2022 Garcia-Moreno, Jordan, Günther, Dau, Fritzsch, Vermes, Schoppa, Ignatius, Wildemann, Werz, Löffler and Tuchscherr.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes.mBio. 2018 Apr 24;9(2):e00415-18. doi: 10.1128/mBio.00415-18. mBio. 2018. PMID: 29691335 Free PMC article.
-
Intracellular Staphylococcus aureus in osteoblasts and osteocytes and its impact on bone homeostasis during osteomyelitis.Bone. 2025 Sep;198:117536. doi: 10.1016/j.bone.2025.117536. Epub 2025 May 18. Bone. 2025. PMID: 40393553 Review.
-
Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation.Int J Med Microbiol. 2014 Nov;304(8):1038-49. doi: 10.1016/j.ijmm.2014.07.013. Epub 2014 Jul 27. Int J Med Microbiol. 2014. PMID: 25129555
-
A Human Osteocyte Cell Line Model for Studying Staphylococcus aureus Persistence in Osteomyelitis.Front Cell Infect Microbiol. 2021 Nov 3;11:781022. doi: 10.3389/fcimb.2021.781022. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34805001 Free PMC article.
-
Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis.Front Cell Infect Microbiol. 2015 Nov 26;5:85. doi: 10.3389/fcimb.2015.00085. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26636047 Free PMC article. Review.
Cited by
-
The endothelium at the interface between tissues and Staphylococcus aureus in the bloodstream.Clin Microbiol Rev. 2025 Mar 13;38(1):e0009824. doi: 10.1128/cmr.00098-24. Epub 2025 Jan 14. Clin Microbiol Rev. 2025. PMID: 39807893 Review.
-
A rapid-release pure iodine coating on titanium implants to mitigate acute periprosthetic infections.Front Bioeng Biotechnol. 2025 Jul 10;13:1590411. doi: 10.3389/fbioe.2025.1590411. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40708857 Free PMC article.
-
Osteomyelitis-relevant antibiotics at clinical concentrations show limited effectivity against acute and chronic intracellular S. aureus infections in osteocytes.Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0080824. doi: 10.1128/aac.00808-24. Epub 2024 Aug 28. Antimicrob Agents Chemother. 2024. PMID: 39194210 Free PMC article.
-
RIG-I and cGAS mediate antimicrobial and inflammatory responses of primary osteoblasts and osteoclasts to Staphylococcus aureus.mBio. 2025 May 14;16(5):e0397124. doi: 10.1128/mbio.03971-24. Epub 2025 Mar 26. mBio. 2025. PMID: 40135931 Free PMC article.
-
The osteoblast secretome in Staphylococcus aureus osteomyelitis.Front Immunol. 2022 Nov 22;13:1048505. doi: 10.3389/fimmu.2022.1048505. eCollection 2022. Front Immunol. 2022. PMID: 36483565 Free PMC article. Review.
References
-
- Ahmed S., Meghji S., Williams R. J., Henderson B., Brock J. H., Nair S. P. (2001). Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69, 2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001, PMID: - DOI - PMC - PubMed
-
- Alder K. D., Lee I., Munger A. M., Kwon H. K., Morris M. T., Cahill S. V., et al. . (2020). Intracellular Staphylococcus aureus in bone and joint infections: a mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone 141:115568. doi: 10.1016/j.bone.2020.115568, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

