Intra-species diversity of Clostridium perfringens: A diverse genetic repertoire reveals its pathogenic potential
- PMID: 35935202
- PMCID: PMC9354469
- DOI: 10.3389/fmicb.2022.952081
Intra-species diversity of Clostridium perfringens: A diverse genetic repertoire reveals its pathogenic potential
Abstract
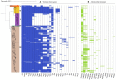
Clostridium perfringens is the causative agent of many enterotoxic diseases in humans and animals, and it is present in diverse environments (soil, food, sewage, and water). Multilocus Sequence Typing (MLST) and Whole Genome Sequencing (WGS) have provided a general approach about genetic diversity of C. perfringens; however, those studies are limited to specific locations and often include a reduced number of genomes. In this study, 372 C. perfringens genomes from multiple locations and sources were used to assess the genetic diversity and phylogenetic relatedness of this pathogen. In silico MLST was used for typing the isolates, and the resulting sequence types (ST) were assigned to clonal complexes (CC) based on allelic profiles that differ from its founder by up to double-locus variants. A pangenome analysis was conducted, and a core genome-based phylogenetic tree was created to define phylogenetic groups. Additionally, key virulence factors, toxinotypes, and antibiotic resistance genes were identified using ABRicate against Virulence Factor Database (VFDB), TOXiper, and Resfinder, respectively. The majority of the C. perfringens genomes found in publicly available databases were derived from food (n = 85) and bird (n = 85) isolates. A total of 195 STs, some of them shared between sources such as food and human, horses and dogs, and environment and birds, were grouped in 25 CC and distributed along five phylogenetic groups. Fifty-three percent of the genomes were allocated to toxinotype A, followed by F (32%) and G (7%). The most frequently found virulence factors based on > 70% coverage and 99.95% identity were plc (100%), nanH (99%), ccp (99%), and colA (98%), which encode an alpha-toxin, a sialidase, an alpha-clostripain, and a collagenase, respectively, while tetA (39.5%) and tetB (36.2%), which mediate tetracycline resistance determinants, were the most common antibiotic resistance genes detected. The analyses conducted here showed a better view of the presence of this pathogen across several host species. They also confirm that the genetic diversity of C. perfringens is based on a large number of virulence factors that vary among phylogroups, and antibiotic resistance markers, especially to tetracyclines, aminoglycosides, and macrolides. Those characteristics highlight the importance of C. perfringens as a one of the most common causes of foodborne illness.
Keywords: Clostridium perfringens; genomic epidemiology; intra-species diversity; multilocus sequence typing; toxinotypes.
Copyright © 2022 Camargo, Guerrero-Araya, Castañeda, Vega, Cardenas-Alvarez, Rodríguez, Paredes-Sabja, Ramírez and Muñoz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comparative genomic analysis of food-animal-derived and human-derived Clostridium perfringens isolates from markets in Shandong, China.Front Microbiol. 2025 Apr 1;16:1543511. doi: 10.3389/fmicb.2025.1543511. eCollection 2025. Front Microbiol. 2025. PMID: 40236475 Free PMC article.
-
Clonal diversity of Clostridium perfringens human clinical isolates with various toxin gene profiles based on multilocus sequence typing and alpha-toxin (PLC) typing.Anaerobe. 2021 Dec;72:102473. doi: 10.1016/j.anaerobe.2021.102473. Epub 2021 Nov 4. Anaerobe. 2021. PMID: 34743038
-
Analysis on characteristics and multilocus sequence typing of Clostridium perfringens in western China.J Antimicrob Chemother. 2025 Jan 3;80(1):216-226. doi: 10.1093/jac/dkae399. J Antimicrob Chemother. 2025. PMID: 39545426
-
Clostridium perfringens-Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance.Pathogens. 2023 May 26;12(6):768. doi: 10.3390/pathogens12060768. Pathogens. 2023. PMID: 37375458 Free PMC article. Review.
-
Genomic analyses of Clostridium perfringens isolates from five toxinotypes.Res Microbiol. 2015 May;166(4):255-63. doi: 10.1016/j.resmic.2014.10.003. Epub 2014 Oct 16. Res Microbiol. 2015. PMID: 25445567 Review.
Cited by
-
Genome MLST scheme for tracing genetic diversity and multidrug resistance of food animal-derived Clostridium perfringens.Curr Res Food Sci. 2025 Jul 20;11:101149. doi: 10.1016/j.crfs.2025.101149. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40735630 Free PMC article.
-
MosAIC: An annotated collection of mosquito-associated bacteria with high-quality genome assemblies.PLoS Biol. 2024 Nov 15;22(11):e3002897. doi: 10.1371/journal.pbio.3002897. eCollection 2024 Nov. PLoS Biol. 2024. PMID: 39546548 Free PMC article.
-
Comparative genomic analysis of food-animal-derived and human-derived Clostridium perfringens isolates from markets in Shandong, China.Front Microbiol. 2025 Apr 1;16:1543511. doi: 10.3389/fmicb.2025.1543511. eCollection 2025. Front Microbiol. 2025. PMID: 40236475 Free PMC article.
-
Particular genomic and virulence traits associated with preterm infant-derived toxigenic Clostridium perfringens strains.Nat Microbiol. 2023 Jun;8(6):1160-1175. doi: 10.1038/s41564-023-01385-z. Epub 2023 May 25. Nat Microbiol. 2023. PMID: 37231089 Free PMC article.
-
Deadly Diarrhea Caused by Co-Infection of Clostridium perfringens Type ST-865 and Clostridium difficile: A Case Report and Review of the Literature.Infect Drug Resist. 2025 Jul 1;18:3231-3236. doi: 10.2147/IDR.S529341. eCollection 2025. Infect Drug Resist. 2025. PMID: 40625918 Free PMC article.
References
-
- Abdel-Glil M. Y., Thomas P., Linde J., Busch A., Wieler L. H., Neubauer H., et al. . (2021). Comparative in silico genome analysis of Clostridium perfringens unravels stable phylogroups with different genome characteristics and pathogenic potential. Sci. Rep. 11:6756. doi: 10.1038/s41598-021-86148-8, PMID: - DOI - PMC - PubMed
-
- Al-Shukri M. S., Hmood A. M., Al-Charrakh A. H. (2021). Sequencing of Clostridium perfringens toxin genes (cpa, etx, iap) from Iraqi hospitals and detection by PCR of the genes encoding resistance to metronidazole, tetracycline, and clindamycin. Indian J. Med. Microbiol. 39, 289–294. doi: 10.1016/j.ijmmb.2021.03.017, PMID: - DOI - PubMed
-
- Awad M. M., Bryant A. E., Stevens D. L., Rood J. I. (1995). Virulence studies on chromosomal α-toxin and Θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15, 191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

