Benchmark Density Functional Theory Approach for the Calculation of Bond Dissociation Energies of the M-O2 Bond: A Key Step in Water Splitting Reactions
- PMID: 35935283
- PMCID: PMC9348009
- DOI: 10.1021/acsomega.2c01331
Benchmark Density Functional Theory Approach for the Calculation of Bond Dissociation Energies of the M-O2 Bond: A Key Step in Water Splitting Reactions
Abstract
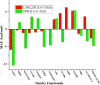
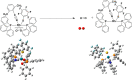
A very fascinating aspect in quantum chemical research is to determine the accurate and cost-effective methods for the calculation of electronic and structural properties through a benchmark study. The current study focuses on the performance evaluation of density functional theory methods for the accurate measurement of bond dissociation energies (BDEs) of chemically important M-O2 bonds in water splitting reactions. The BDE measurement has got noteworthy attention due to its importance in all areas of chemistry. For BDE measurements of M-O2 bonds in five metal complexes with oxygen molecules, 14 density functionals (DFs) are chosen from seven classes of DFs with two series of mixed basis sets. A combination of pseudopotential and Pople basis sets [LANL2DZ & 6-31G(d) and SDD & 6-31+G(d)] are used as a series of mixed basis sets. The B3LYP-GD3BJ functional with LANL2DZ & 6-31G(d) gives outstanding results due to low deviations, error, and the best Pearson's correlation (R) between the experimental and theoretical data. Our study suggested an efficient, low-cost, precise, and accurate B3LYP-GD3BJ/LANL2DZ & 6-31G(d) level of theory for BDE of the M-O2 bond, which may be useful for chemists working in the field of energy generation and utilization.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Shahbaz M.; Mutascu M.; Azim P. Environmental Kuznets Curve in Romania and the Role of Energy Consumption. Renewable Sustainable Energy Rev. 2013, 18, 165–173. 10.1016/j.rser.2012.10.012. - DOI
-
- Bard A. J.; Fox M. A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. 10.1021/ar00051a007. - DOI
LinkOut - more resources
Full Text Sources
