Effects of 3-methylmethcathinone on conditioned place preference and anxiety-like behavior: Comparison with methamphetamine
- PMID: 35935336
- PMCID: PMC9354685
- DOI: 10.3389/fnmol.2022.975820
Effects of 3-methylmethcathinone on conditioned place preference and anxiety-like behavior: Comparison with methamphetamine
Abstract
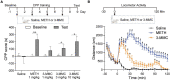
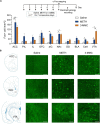
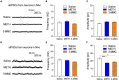
3-Methylmethcathinone (3-MMC), a drug belonging to synthetic cathinones family, raised public attention due to its harmful health effects and abuse potential. Although it has similar properties to other cathinone derivatives, the behavioral effects of 3-MMC remain largely unknown. In the present research, we evaluated the rewarding effect of 3-MMC using conditioned place preference (CPP) paradigm and its effect on anxiety-like behavior using elevated plus maze (EPM) and compared with methamphetamine (METH). Then, we performed a whole-brain c-Fos mapping to identify the specific brain regions in response to 3-MMC exposure and explored the changes of synaptic transmission in nucleus accumbens (NAc) using patch-clamp recording after chronic 3-MMC and METH exposure. 3-MMC induced CPP at higher doses of 3 or 10 mg/kg in rats and acute exposure of 3 mg/kg 3-MMC to rats produced anxiolytic-like effect, while anxiety-like behavior was increased after 7 days of injection with 3-MMC. Whole-brain immunostaining revealed increased c-Fos expression in anterior cingulate cortex (ACC), NAc and ventral tegmental area (VTA) after chronic 3-MMC injection compared with saline, which was similar to METH. Especially, 3-MMC induced more neural activation of VTA compared with METH. Finally, we found that amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) in NAc was decreased after chronic 3-MMC injection, while frequency of sIPSCs and spontaneous excitatory postsynaptic currents (sEPSCs) were not affected. Taken together, our results revealed the addictive potential of 3-MMC and its effect on anxiety-like behavior, which warn the risks of 3-MMC abuse and justify the control of synthetic cathinones. And 3-MMC selectively inhibit inhibitory but not excitatory transmission onto neurons in NAc, which may contribute to its effects.
Keywords: 3-methylmethcathinone; conditioned place preference; elevated plus maze; nucleus accumbens; synaptic transmission.
Copyright © 2022 Chen, Zhang, Ding, Wu, Wang and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Methamphetamine Activates Toll-Like Receptor 4 to Induce Central Immune Signaling within the Ventral Tegmental Area and Contributes to Extracellular Dopamine Increase in the Nucleus Accumbens Shell.ACS Chem Neurosci. 2019 Aug 21;10(8):3622-3634. doi: 10.1021/acschemneuro.9b00225. Epub 2019 Jul 17. ACS Chem Neurosci. 2019. PMID: 31282647 Free PMC article.
-
Neural Circuits Associated with 5-HT1B Receptor Agonist Inhibition of Methamphetamine Seeking in the Conditioned Place Preference Model.ACS Chem Neurosci. 2019 Jul 17;10(7):3271-3283. doi: 10.1021/acschemneuro.8b00709. Epub 2019 May 29. ACS Chem Neurosci. 2019. PMID: 31042352
-
Manganese-enhanced magnetic resonance imaging reveals differential long-term neuroadaptation after methamphetamine and the substituted cathinone 4-methylmethcathinone (mephedrone).Int J Neuropsychopharmacol. 2014 Dec 7;18(6):pyu106. doi: 10.1093/ijnp/pyu106. Int J Neuropsychopharmacol. 2014. PMID: 25522432 Free PMC article.
-
Ameliorative effects of alkaloid extract from Mitragyna speciosa (Korth.) Havil. Leaves on methamphetamine conditioned place preference in mice.J Ethnopharmacol. 2022 Feb 10;284:114824. doi: 10.1016/j.jep.2021.114824. Epub 2021 Nov 8. J Ethnopharmacol. 2022. PMID: 34763040
-
Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies.Behav Brain Res. 1999 Jun;101(2):129-52. doi: 10.1016/s0166-4328(99)00022-4. Behav Brain Res. 1999. PMID: 10372570 Review.
Cited by
-
3'-Deoxyadenosin alleviates methamphetamine-induced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome.Neural Regen Res. 2024 Oct 1;19(10):2270-2280. doi: 10.4103/1673-5374.392887. Epub 2024 Jan 26. Neural Regen Res. 2024. PMID: 38488561 Free PMC article.
-
An orbitofrontal cortex-anterior insular cortex circuit gates compulsive cocaine use.Sci Adv. 2022 Dec 23;8(51):eabq5745. doi: 10.1126/sciadv.abq5745. Epub 2022 Dec 23. Sci Adv. 2022. PMID: 36563158 Free PMC article.
-
Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile.Medicina (Kaunas). 2024 Mar 12;60(3):466. doi: 10.3390/medicina60030466. Medicina (Kaunas). 2024. PMID: 38541192 Free PMC article. Review.
-
Distinct role of claustrum and anterior cingulate cortex bidirectional circuits in methamphetamine taking and seeking.Nat Commun. 2025 Jul 25;16(1):6871. doi: 10.1038/s41467-025-62188-w. Nat Commun. 2025. PMID: 40715089 Free PMC article.
-
Quantifying conditioned place preference: a review of current analyses and a proposal for a novel approach.Front Behav Neurosci. 2023 Aug 24;17:1256764. doi: 10.3389/fnbeh.2023.1256764. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37693282 Free PMC article.
References
LinkOut - more resources
Full Text Sources

