A switchable light-responsive azopolymer conjugating protein micropatterns with topography for mechanobiological studies
- PMID: 35935479
- PMCID: PMC9355574
- DOI: 10.3389/fbioe.2022.933410
A switchable light-responsive azopolymer conjugating protein micropatterns with topography for mechanobiological studies
Abstract
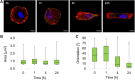
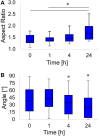
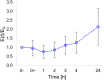
Stem cell shape and mechanical properties in vitro can be directed by geometrically defined micropatterned adhesion substrates. However, conventional methods are limited by the fixed micropattern design, which cannot recapitulate the dynamic changes of the natural cell microenvironment. Current methods to fabricate dynamic platforms usually rely on complex chemical strategies or require specialized apparatuses. Also, with these methods, the integration of dynamic signals acting on different length scales is not straightforward, whereas, in some applications, it might be beneficial to act on both a microscale level, that is, cell shape, and a nanoscale level, that is, cell adhesions. Here, we exploited a confocal laser-based technique on a light-responsive azopolymer displaying micropatterns of adhesive islands. The laser light promotes a directed mass migration and the formation of submicrometric topographic relieves. Also, by changing the surface chemistry, the surfacing topography affects cell spreading and shape. This method enabled us to monitor in a non-invasive manner the dynamic changes in focal adhesions, cytoskeleton structures, and nucleus conformation that followed the changes in the adhesive characteristic of the substrate. Focal adhesions reconfigured after the surfacing of the topography, and the actin filaments reoriented to coalign with the newly formed adhesive island. Changes in cell morphology also affected nucleus shape, chromatin conformation, and cell mechanics with different timescales. The reported strategy can be used to investigate mechanotransduction-related events dynamically by controlling cell adhesion at cell shape and focal adhesion levels. The integrated technique enables achieving a submicrometric resolution in a facile and cost-effective manner.
Keywords: azopolymer; cell adhesion; cell shape; dynamic substrates; light-responsive; mechanotransduction; nucleus shape.
Copyright © 2022 Cimmino, Netti and Ventre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GB declared a past co-authorship with the authors PN and MV to the handling editor.
Figures



Similar articles
-
Micropatterned Azopolymer Surfaces Modulate Cell Mechanics and Cytoskeleton Structure.ACS Appl Mater Interfaces. 2015 Sep 30;7(38):21503-10. doi: 10.1021/acsami.5b06693. Epub 2015 Sep 21. ACS Appl Mater Interfaces. 2015. PMID: 26372777
-
Reversible Holographic Patterns on Azopolymers for Guiding Cell Adhesion and Orientation.ACS Appl Mater Interfaces. 2015 Aug 12;7(31):16984-91. doi: 10.1021/acsami.5b02080. Epub 2015 Jul 28. ACS Appl Mater Interfaces. 2015. PMID: 25876082
-
Substrate micropatterns produced by polymer demixing regulate focal adhesions, actin anisotropy, and lineage differentiation of stem cells.Acta Biomater. 2018 Aug;76:21-28. doi: 10.1016/j.actbio.2018.06.020. Epub 2018 Jun 12. Acta Biomater. 2018. PMID: 29906627 Free PMC article.
-
Spatio-Temporal Control of Cell Adhesion: Toward Programmable Platforms to Manipulate Cell Functions and Fate.Front Bioeng Biotechnol. 2018 Dec 4;6:190. doi: 10.3389/fbioe.2018.00190. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30564573 Free PMC article. Review.
-
Dynamic azopolymeric interfaces for photoactive cell instruction.Biophys Rev (Melville). 2020 Nov 16;1(1):011302. doi: 10.1063/5.0025175. eCollection 2020 Dec. Biophys Rev (Melville). 2020. PMID: 38505629 Free PMC article. Review.
Cited by
-
Assessing the stability of azopolymer nanotopography during live-cell fluorescence imaging.Front Bioeng Biotechnol. 2024 Aug 13;12:1409735. doi: 10.3389/fbioe.2024.1409735. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39193229 Free PMC article.
-
Biomimetic Cardiac Tissue Models for In Vitro Arrhythmia Studies.Biomimetics (Basel). 2023 Oct 14;8(6):487. doi: 10.3390/biomimetics8060487. Biomimetics (Basel). 2023. PMID: 37887618 Free PMC article. Review.
-
Cell biomechanics on muscle atrophy: from intricate mechanisms to therapeutic frontiers.Ann Med. 2025 Dec;57(1):2540598. doi: 10.1080/07853890.2025.2540598. Epub 2025 Aug 1. Ann Med. 2025. PMID: 40747836 Free PMC article. Review.
-
Shape-Morphing Photoresponsive Hydrogels Reveal Dynamic Topographical Conditioning of Fibroblasts.Adv Sci (Weinh). 2023 Nov;10(31):e2303136. doi: 10.1002/advs.202303136. Epub 2023 Sep 23. Adv Sci (Weinh). 2023. PMID: 37740666 Free PMC article.
References
-
- Anderson H. J., Sahoo J. K., Ulijn R. V., Dalby M. J. (2016). Mesenchymal stem cell fate: applying biomaterials for control of stem cell behavior. Front. Bioeng. Biotechnol. 4, 38. 10.3389/fbioe.2016.00038 PubMed Abstract | 10.3389/fbioe.2016.00038 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Cimmino C., Rossano L., Netti P. A., Ventre M. (2018). Spatio-temporal control of cell adhesion: toward programmable platforms to manipulate cell functions and fate. Front. Bioeng. Biotechnol. 6, 190. 10.3389/fbioe.2018.00190 PubMed Abstract | 10.3389/fbioe.2018.00190 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Damodaran K., Venkatachalapathy S., Alisafaei F., Radhakrishnan A. V., Sharma Jokhun D., Shenoy V. B., et al. (2018). Compressive force induces reversible chromatin condensation and cell geometry-dependent transcriptional response. Mol. Biol. Cell 29, 3039–3051. 10.1091/mbc.E18-04-0256 PubMed Abstract | 10.1091/mbc.E18-04-0256 | Google Scholar - DOI - DOI - PMC - PubMed
-
- De Martino S., Cavalli S., Netti P. A. (2020). Photoactive interfaces for spatio-temporal guidance of mesenchymal stem cell fate. Adv. Healthc. Mat. 9, 2000470. 10.1002/adhm.202000470 10.1002/adhm.202000470 | Google Scholar - DOI - DOI - PubMed
-
- Egeblad M., Rasch M. G., Weaver V. M. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22, 697–706. 10.1016/j.ceb.2010.08.015 PubMed Abstract | 10.1016/j.ceb.2010.08.015 | Google Scholar - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

