Directed Self-Assembly of Heterologously Expressed Hagfish EsTKα and EsTKγ for Functional Hydrogel
- PMID: 35935505
- PMCID: PMC9354048
- DOI: 10.3389/fbioe.2022.960586
Directed Self-Assembly of Heterologously Expressed Hagfish EsTKα and EsTKγ for Functional Hydrogel
Abstract
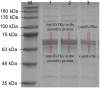
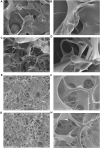
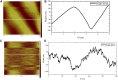
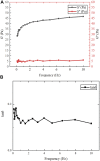
Hagfish slime proteins have long been considered useful due to their potential applications in novel green, environmental, and functional bionic materials. The two main component proteins in the slime thread of hagfish, (opt)EsTKα and (opt)EsTKγ, were used as raw materials. However, the methods available to assemble these two proteins are time- and labor-intensive. The conditions affecting protein self-assembly, such as the pH of the assembly buffer, protein concentration, and the protein addition ratio, were the subject of the present research. Through a series of tests, the self-assembly results of a variety of assembly conditions were explored. Finally, a simplified protein self-assembly method was identified that allows for simple, direct assembly of the two proteins directly. This method does not require protein purification. Under the optimal assembly conditions obtained by exploration, a new gel material was synthesized from the hagfish protein through self-assembly of the (opt)EsTKα and (opt)EsTKγ. This assembly method has the benefits of being a simple, time-saving, and efficient. The self-assembled protein gel products were verified by SDS polyacrylamide gel electrophoresis (SDS-PAGE) and contained (opt)EsTKα and (opt)EsTKγ proteins. Scanning electron microscopy (SEM) was used to investigate the self-assembled protein gel after freeze-drying, and it was observed that the self-assembled protein formed a dense, three-dimensional porous network structure, meaning that it had good water retention. Evaluation of the gel with atomic force microscopy (AFM) indicated that the surface of the protein fiber skeleton show the network-like structure and relatively smooth. Characterization by circular dichroism (CD) and Fourier transform infrared spectroscopy (FT-IR) demonstrated that the two proteins were successfully assembled, and that the assembled protein had a secondary structure dominated by α-helices. The rheological properties of the self-assembled products were tested to confirm that they were indeed hydrogel property.
Keywords: hagfish slime protein; isolation; phase separation; purification; recombinant protein; self-assembly.
Copyright © 2022 Sun, Zheng, Zhu, Zhou, Liu and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Self-Assembly of Recombinant Hagfish Thread Keratins Amenable to a Strain-Induced α-Helix to β-Sheet Transition.Biomacromolecules. 2015 Aug 10;16(8):2327-39. doi: 10.1021/acs.biomac.5b00552. Epub 2015 Jul 8. Biomacromolecules. 2015. PMID: 26102237
-
Concentration-independent mechanics and structure of hagfish slime.Acta Biomater. 2018 Oct 1;79:123-134. doi: 10.1016/j.actbio.2018.08.022. Epub 2018 Aug 29. Acta Biomater. 2018. PMID: 30170194
-
Fiber-Enforced Hydrogels: Hagfish Slime Stabilized with Biopolymers including κ-Carrageenan.ACS Biomater Sci Eng. 2016 Jan 11;2(1):90-95. doi: 10.1021/acsbiomaterials.5b00404. Epub 2015 Nov 25. ACS Biomater Sci Eng. 2016. PMID: 33418646
-
Structure and Nanomechanics of Dry and Hydrated Intermediate Filament Films and Fibers Produced from Hagfish Slime Fibers.ACS Appl Mater Interfaces. 2018 Nov 28;10(47):40460-40473. doi: 10.1021/acsami.8b17166. Epub 2018 Nov 14. ACS Appl Mater Interfaces. 2018. PMID: 30371056
-
From reductionism to synthesis: The case of hagfish slime.Comp Biochem Physiol B Biochem Mol Biol. 2021 Aug-Sep;255:110610. doi: 10.1016/j.cbpb.2021.110610. Epub 2021 May 7. Comp Biochem Physiol B Biochem Mol Biol. 2021. PMID: 33971350 Review.
Cited by
-
Targeted PHA Microsphere-Loaded Triple-Drug System with Sustained Drug Release for Synergistic Chemotherapy and Gene Therapy.Nanomaterials (Basel). 2024 Oct 16;14(20):1657. doi: 10.3390/nano14201657. Nanomaterials (Basel). 2024. PMID: 39452993 Free PMC article.
References
-
- Cheng Y., Donkor P. O., Ren X., Wu J., Agyemang K., Ayim I., et al. (2019). Effect of Ultrasound Pretreatment with Mono-Frequency and Simultaneous Dual Frequency on the Mechanical Properties and Microstructure of Whey Protein Emulsion Gels. Food Hydrocoll. 89, 434–442. 10.1016/j.foodhyd.2018.11.007 - DOI
-
- Dong X., Yang X., Li H., Che H., Song L., Chen X., et al. (2021). Effects of Oxidation on the Structure of Collagen Fibers of Sea Cucumber (Apostichopus Japonicus) Body Wall during Thermal Processing. LWT- Food Sci. Technol. 138, 110528. 10.1016/j.lwt.2020.110528 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

