Transfer mechanism of cell-free synthesized membrane proteins into mammalian cells
- PMID: 35935506
- PMCID: PMC9355040
- DOI: 10.3389/fbioe.2022.906295
Transfer mechanism of cell-free synthesized membrane proteins into mammalian cells
Abstract
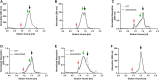
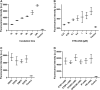
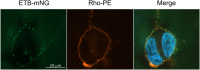
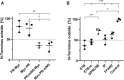
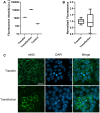
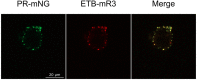
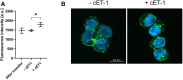
Nanodiscs are emerging to serve as transfer vectors for the insertion of recombinant membrane proteins into membranes of living cells. In combination with cell-free expression technologies, this novel process opens new perspectives to analyze the effects of even problematic targets such as toxic, hard-to-express, or artificially modified membrane proteins in complex cellular environments of different cell lines. Furthermore, transferred cells must not be genetically engineered and primary cell lines or cancer cells could be implemented as well. We have systematically analyzed the basic parameters of the nanotransfer approach and compared the transfer efficiencies from nanodiscs with that from Salipro particles. The transfer of five membrane proteins was analyzed: the prokaryotic proton pump proteorhodopsin, the human class A family G-protein coupled receptors for endothelin type B, prostacyclin, free fatty acids type 2, and the orphan GPRC5B receptor as a class C family member. The membrane proteins were cell-free synthesized with a detergent-free strategy by their cotranslational insertion into preformed nanoparticles containing defined lipid environments. The purified membrane protein/nanoparticles were then incubated with mammalian cells. We demonstrate that nanodiscs disassemble and only lipids and membrane proteins, not the scaffold protein, are transferred into cell membranes. The process is detectable within minutes, independent of the nanoparticle lipid composition, and the transfer efficiency directly correlates with the membrane protein concentration in the transfer mixture and with the incubation time. Transferred membrane proteins insert in both orientations, N-terminus in and N-terminus out, in the cell membrane, and the ratio can be modulated by engineering. The viability of cells is not notably affected by the transfer procedure, and transferred membrane proteins stay detectable in the cell membrane for up to 3 days. Transferred G-protein coupled receptors retained their functionality in the cell environment as shown by ligand binding, induction of internalization, and specific protein interactions. In comparison to transfection, the cellular membrane protein concentration is better controllable and more uniformly distributed within the analyzed cell population. A further notable difference to transfection is the accumulation of transferred membrane proteins in clusters, presumably determined by microdomain structures in the cell membranes.
Keywords: G-protein coupled receptors; GPCR function; HEK 293 cell; Salipro nanoparticles; cell-free expression; nanodiscs; protein transfer; transfection.
Copyright © 2022 Umbach, Levin, Neumann, Steinmetzer, Dötsch and Bernhard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Cotranslational assembly of membrane protein/nanoparticles in cell-free systems.Biochim Biophys Acta Biomembr. 2022 Nov 1;1864(11):184017. doi: 10.1016/j.bbamem.2022.184017. Epub 2022 Jul 31. Biochim Biophys Acta Biomembr. 2022. PMID: 35921875
-
Functional properties of cell-free expressed human endothelin A and endothelin B receptors in artificial membrane environments.Biochim Biophys Acta. 2013 Sep;1828(9):2182-92. doi: 10.1016/j.bbamem.2013.05.031. Epub 2013 Jun 6. Biochim Biophys Acta. 2013. PMID: 23747296
-
Biochemical Characterization of Cell-free Synthesized Human β1 Adrenergic Receptor Cotranslationally Inserted into Nanodiscs.J Mol Biol. 2022 Aug 30;434(16):167687. doi: 10.1016/j.jmb.2022.167687. Epub 2022 Jun 16. J Mol Biol. 2022. PMID: 35717996
-
Cell-Free Co-Translational Approaches for Producing Mammalian Receptors: Expanding the Cell-Free Expression Toolbox Using Nanolipoproteins.Front Pharmacol. 2019 Jul 3;10:744. doi: 10.3389/fphar.2019.00744. eCollection 2019. Front Pharmacol. 2019. PMID: 31333463 Free PMC article. Review.
-
Polymer Nanodiscs and Their Bioanalytical Potential.Chemistry. 2021 Sep 9;27(51):12922-12939. doi: 10.1002/chem.202101572. Epub 2021 Jul 30. Chemistry. 2021. PMID: 34180107 Review.
Cited by
-
Cryo-EM structure of cell-free synthesized human histamine 2 receptor/Gs complex in nanodisc environment.Nat Commun. 2024 Feb 28;15(1):1831. doi: 10.1038/s41467-024-46096-z. Nat Commun. 2024. PMID: 38418462 Free PMC article.
References
-
- Carvalho J., Chennupati R., Li R., Günther S., Kaur H., Zhao W., et al. (2020). Orphan G protein-coupled receptor GPRC5B controls smooth muscle contractility and differentiation by inhibiting prostacyclin receptor signaling. Circulation 141, 1168–1183. 10.1161/circulationaha.119.043703 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

