Guanxinning Tablet Attenuates Coronary Atherosclerosis via Regulating the Gut Microbiota and Their Metabolites in Tibetan Minipigs Induced by a High-Fat Diet
- PMID: 35935588
- PMCID: PMC9352486
- DOI: 10.1155/2022/7128230
Guanxinning Tablet Attenuates Coronary Atherosclerosis via Regulating the Gut Microbiota and Their Metabolites in Tibetan Minipigs Induced by a High-Fat Diet
Abstract
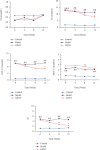
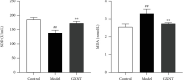
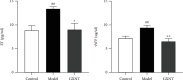
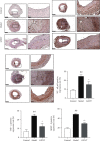
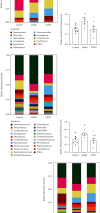
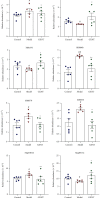
Coronary atherosclerosis (CA) is a chronic and evolving inflammatory disease characterized by the build-up of atherosclerotic plaque in the wall of coronary arteries. Guanxinning tablet (GXNT) is a novel Chinese medicine formula, which has been clinically used to treat coronary heart disease for many years. However, the potential mechanism for treating CA remains unclear. Thus, the study was aimed at investigating the therapeutic effect of GXNT on CA and further explore the underlying mechanisms from the perspective of gut microbiota. Following the establishment of a CA model in Tibetan minipigs, GXNT was orally administrated. We simultaneously detected blood lipid levels, observed ventricular function using ultrasound examination, measured platelet aggregation, and checked changes in inflammatory factors, oxidative stress factors, and vascular endothelial injury-related indexes applying ELISA assays. Histopathological changes of coronary artery tissue were subsequently evaluated using Sudan IV staining, HE staining, Oil red "O" staining, and immunohistochemistry assays. Finally, alterations of the gut microbiota and microbial metabolites were detected using metagenomic sequencing and targeted metabolomics, respectively. The results have suggested that GXNT could regulate dyslipidemia, improve heart function, and inhibit the levels of ox-LDL, CRP, TNF-α, IL-1β, SOD, MDA, vWF, and ET-1, as well as platelet aggregation. Additionally, histopathological findings revealed that GXNT could reduce lipid deposition, alleviate AS lesions, and restrain the expressions of NF-κB, TNF-α, and MMP-9. Furthermore, the composition of the gut microbiota was altered. Specifically, GXNT could upregulate the relative abundance of Prevotellaceae and Prevotella and downregulate the abundance of Proteobacteria, Enterobacteriaceae, and Escherichia. As for microbial metabolites, GXNT could increase fecal propionic acid, butyric acid, and LCA-3S and decrease fecal TMA-related metabolites, CDCA, and serum TMAO. In sum, the results showed that GXNT had a satisfactory anti-CA effect, and the mechanism was closely associated with modulating gut microbiota and related metabolites.
Copyright © 2022 Qinqin Yang et al.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

