Automated analysis of limited echocardiograms: Feasibility and relationship to outcomes in COVID-19
- PMID: 35935624
- PMCID: PMC9353267
- DOI: 10.3389/fcvm.2022.937068
Automated analysis of limited echocardiograms: Feasibility and relationship to outcomes in COVID-19
Abstract
Background: As automated echocardiographic analysis is increasingly utilized, continued evaluation within hospital settings is important to further understand its potential value. The importance of cardiac involvement in patients hospitalized with COVID-19 provides an opportunity to evaluate the feasibility and clinical relevance of automated analysis applied to limited echocardiograms.
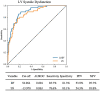
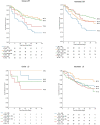
Methods: In this multisite US cohort, the feasibility of automated AI analysis was evaluated on 558 limited echocardiograms in patients hospitalized with COVID-19. Reliability of automated assessment of left ventricular (LV) volumes, ejection fraction (EF), and LV longitudinal strain (LS) was assessed against clinically obtained measures and echocardiographic findings. Automated measures were evaluated against patient outcomes using ROC analysis, survival modeling, and logistic regression for the outcomes of 30-day mortality and in-hospital sequelae.
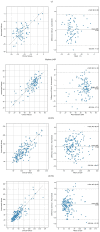
Results: Feasibility of automated analysis for both LVEF and LS was 87.5% (488/558 patients). AI analysis was performed with biplane method in 300 (61.5%) and single plane apical 4- or 2-chamber analysis in 136 (27.9%) and 52 (10.7%) studies, respectively. Clinical LVEF was assessed using visual estimation in 192 (39.3%), biplane in 163 (33.4%), and single plane or linear methods in 104 (21.2%) of the 488 studies; 29 (5.9%) studies did not have clinically reported LVEF. LV LS was clinically reported in 80 (16.4%). Consistency between automated and clinical values demonstrated Pearson's R, root mean square error (RMSE) and intraclass correlation coefficient (ICC) of 0.61, 11.3% and 0.72, respectively, for LVEF; 0.73, 3.9% and 0.74, respectively for LS; 0.76, 24.4ml and 0.87, respectively, for end-diastolic volume; and 0.82, 12.8 ml, and 0.91, respectively, for end-systolic volume. Abnormal automated measures of LVEF and LS were associated with LV wall motion abnormalities, left atrial enlargement, and right ventricular dysfunction. Automated analysis was associated with outcomes, including survival.
Conclusion: Automated analysis was highly feasible on limited echocardiograms using abbreviated protocols, consistent with equivalent clinically obtained metrics, and associated with echocardiographic abnormalities and patient outcomes.
Keywords: COVID-19; artificial intelligence; deformation imaging; echocardiography; machine learning; strain rate imaging.
Copyright © 2022 Pellikka, Strom, Pajares-Hurtado, Keane, Khazan, Qamruddin, Tutor, Gul, Peterson, Thamman, Watson, Mandale, Scott, Naqvi, Woodward and Hawkes.
Figures
Similar articles
-
Single-Site Experience with an Automated Artificial Intelligence Application for Left Ventricular Ejection Fraction Measurement in Echocardiography.Diagnostics (Basel). 2023 Mar 30;13(7):1298. doi: 10.3390/diagnostics13071298. Diagnostics (Basel). 2023. PMID: 37046515 Free PMC article.
-
Automated Echocardiographic Quantification of Left Ventricular Ejection Fraction Without Volume Measurements Using a Machine Learning Algorithm Mimicking a Human Expert.Circ Cardiovasc Imaging. 2019 Sep;12(9):e009303. doi: 10.1161/CIRCIMAGING.119.009303. Epub 2019 Sep 16. Circ Cardiovasc Imaging. 2019. PMID: 31522550 Free PMC article.
-
Feasibility of New Transthoracic Three-Dimensional Echocardiographic Automated Software for Left Heart Chamber Quantification in Children.J Am Soc Echocardiogr. 2019 Jan;32(1):121-134.e1. doi: 10.1016/j.echo.2018.08.001. Epub 2018 Sep 18. J Am Soc Echocardiogr. 2019. PMID: 30241929
-
Validity of automated measurement of left ventricular ejection fraction and volume using the Philips EPIQ system.Echocardiography. 2017 Nov;34(11):1575-1583. doi: 10.1111/echo.13705. Epub 2017 Oct 10. Echocardiography. 2017. PMID: 28994128
-
Myocardial strain to detect subtle left ventricular systolic dysfunction.Eur J Heart Fail. 2017 Mar;19(3):307-313. doi: 10.1002/ejhf.694. Epub 2016 Nov 27. Eur J Heart Fail. 2017. PMID: 27891719 Review.
Cited by
-
Single-Site Experience with an Automated Artificial Intelligence Application for Left Ventricular Ejection Fraction Measurement in Echocardiography.Diagnostics (Basel). 2023 Mar 30;13(7):1298. doi: 10.3390/diagnostics13071298. Diagnostics (Basel). 2023. PMID: 37046515 Free PMC article.
-
Artificial intelligence-based classification of echocardiographic views.Eur Heart J Digit Health. 2024 Feb 26;5(3):260-269. doi: 10.1093/ehjdh/ztae015. eCollection 2024 May. Eur Heart J Digit Health. 2024. PMID: 38774376 Free PMC article.
-
Point-of-Care Ultrasonography in the Critical Care Unit: An Update.Curr Cardiol Rep. 2025 Feb 15;27(1):54. doi: 10.1007/s11886-024-02187-3. Curr Cardiol Rep. 2025. PMID: 39954172 Free PMC article. Review.
References
-
- Shahin AI, Almotairi S. An accurate and fast cardio-views classification system based on fused deep features and LSTM. IEEE Access. (2020) 8:135184–94. 10.1109/ACCESS.2020.3010326 - DOI
-
- Van Woudenberg N, Liao Z, Abdi AH, Girgis H, Luong C, Vaseli H, et al. . Quantitative echocardiography: Real-time quality estimation and view classification implemented on a mobile android device. In: Simulation, Image Processing, and Ultrasound Systems for Assisted Diagnosis and Navigation. 2018. Lecture Notes in Computer Science. Cham: Springer; (2018) 11042, p. 74–81. 10.1007/978-3-030-01045-4_9 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

