A matter of delicate balance: Loss and gain of Cockayne syndrome proteins in premature aging and cancer
- PMID: 35935726
- PMCID: PMC9351357
- DOI: 10.3389/fragi.2022.960662
A matter of delicate balance: Loss and gain of Cockayne syndrome proteins in premature aging and cancer
Abstract
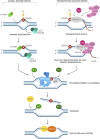
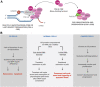
DNA repair genes are critical for preserving genomic stability and it is well established that mutations in DNA repair genes give rise to progeroid diseases due to perturbations in different DNA metabolic activities. Cockayne Syndrome (CS) is an autosomal recessive inheritance caused by inactivating mutations in CSA and CSB genes. This review will primarily focus on the two Cockayne Syndrome proteins, CSA and CSB, primarily known to be involved in Transcription Coupled Repair (TCR). Curiously, dysregulated expression of CS proteins has been shown to exhibit differential health outcomes: lack of CS proteins due to gene mutations invariably leads to complex premature aging phenotypes, while excess of CS proteins is associated with carcinogenesis. Thus it appears that CS genes act as a double-edged sword whose loss or gain of expression leads to premature aging and cancer. Future mechanistic studies on cell and animal models of CS can lead to potential biological targets for interventions in both aging and cancer development processes. Some of these exciting possibilities will be discussed in this review in light of the current literature.
Keywords: Cockayne Syndrome; aging; cancer; premature aging syndromes; transcription coupled repair.
Copyright © 2022 Paccosi, Balajee and Proietti-De-Santis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aamann M. D., Sorensen M. M., Hvitby C., Berquist B. R., Muftuoglu M., Tian J., et al. (2010). Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane. FASEB J. 24 (7), 2334–2346. 10.1096/fj.09-147991 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

