The regulatory challenges of innovative customized combination products
- PMID: 35935795
- PMCID: PMC9354569
- DOI: 10.3389/fmed.2022.821094
The regulatory challenges of innovative customized combination products
Abstract
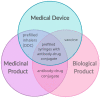
Background/aims: Combination products are therapeutic and/or diagnostic products that can combine drugs and medical devices and which increasing complexity has raised new regulatory framework challenges. To reach the market, a combination product must be classified based on the principal mode of action (PMOA). However, research and technological progress has been leading to the development of novel combination products with no clearly defined PMOA, emphasizing the lack of a systematization process, thus challenging the correct classification of these products. To illustrate the regulatory challenge, two case studies are discussed: innovative combination products with PMOA that can change due to an external stimulus, specifically custom-made 3D-printed scaffolds with incorporated medicinal substances.
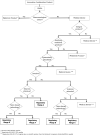
Methods: Data was collected through computational search engines, regulatory agencies and equally relevant associations. The analysis of the data resulted on this state-of-the-art review, a description of the decision-making process by the regulatory authorities, and case studies analysis that culminated in the proposal of a decision-tree scheme.
Findings: Current regulations do not fully address complex combination products namely personalized 3D-printed scaffolds. Two merged regulatory approaches are suggested along with the schematization of the rational assisted by a decision-tree tool.
Conclusion: Combination products have become increasingly sophisticated, which has furthered the need to develop multidisciplinary collaborations within the health sector to adapt to these innovative healthcare solutions as well as with regulators to overcome the challenges posed for their classification.
Keywords: 3D-printed scaffold; combination products; custom-made devices; drug-device; principal mode of action; regulatory aspects.
Copyright © 2022 Reis, Bettencourt and Ribeiro.
Figures

Similar articles
-
Ensuring safety and efficacy in combination products: regulatory challenges and best practices.Front Med Technol. 2024 Jul 10;6:1377443. doi: 10.3389/fmedt.2024.1377443. eCollection 2024. Front Med Technol. 2024. PMID: 39050909 Free PMC article. Review.
-
Regulatory Framework for Drug-Device Combination Products in the United States, Europe, and Korea.Ther Innov Regul Sci. 2024 Sep;58(5):796-806. doi: 10.1007/s43441-024-00661-2. Epub 2024 May 8. Ther Innov Regul Sci. 2024. PMID: 38717522 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Fighting trafficking of falsified and substandard medicinal products in Russia.Int J Risk Saf Med. 2015;27 Suppl 1:S37-40. doi: 10.3233/JRS-150681. Int J Risk Saf Med. 2015. PMID: 26639702
-
Drug-device combination products: regulatory landscape and market growth.Drugs Today (Barc). 2015 Aug;51(8):505-13. doi: 10.1358/dot.2015.51.8.2376223. Drugs Today (Barc). 2015. PMID: 26380388
Cited by
-
Ensuring safety and efficacy in combination products: regulatory challenges and best practices.Front Med Technol. 2024 Jul 10;6:1377443. doi: 10.3389/fmedt.2024.1377443. eCollection 2024. Front Med Technol. 2024. PMID: 39050909 Free PMC article. Review.
-
Hydrogels in Cardiac Surgery: Versatile Platforms for Tissue Repair, Adhesion Prevention, and Localized Therapeutics.Gels. 2025 Jul 21;11(7):564. doi: 10.3390/gels11070564. Gels. 2025. PMID: 40710725 Free PMC article. Review.
References
-
- Morang J, Privitera MB. Combination Devices. Applied Human Factors in Medical Design. Cambridge, MA: Academic Press; (2019).
-
- Gopalaswamy S, Gopalaswamy V. Combination Products: Regulatory Challenges and Successful Product Development. Boca Raton, FL: CRC Press, Taylor & Francis Group; (2008). p. 221–4.
-
- Medtech Europe . Innovation in Medical Technologies – Reflection Paper. Available online at: https://medtecheurope.org/resource-library/innovation-in-medical-technol... (accessed October 19, 2020).
LinkOut - more resources
Full Text Sources

