Case Report: Subacute thyroiditis after receiving inactivated SARS-CoV-2 vaccine (BBIBP-CorV)
- PMID: 35935798
- PMCID: PMC9355607
- DOI: 10.3389/fmed.2022.918721
Case Report: Subacute thyroiditis after receiving inactivated SARS-CoV-2 vaccine (BBIBP-CorV)
Abstract
Background: Subacute thyroiditis, an inflammatory disease, has been reported caused by vaccines in rare cases. In the context of the coronavirus disease 19 pandemic, various SARS-CoV-2 vaccines have been developed and may be potential triggers for subacute thyroiditis.
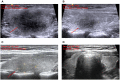
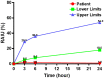
Case presentation: We report a case of subacute thyroiditis 3 days after receiving the second dose of inactivated SARS-CoV-2 vaccine (BBIBP-CorV). The patient did not report a previous history of thyroid disease, upper respiratory tract infection, or COVID-19. Physical examination, laboratory testing, ultrasonography, and radioactive iodine uptake were consistent with subacute thyroiditis. During follow-up, the patient recovered from symptoms and signs, and imaging changes except for hypothyroidism, requiring an ongoing thyroxine replacement.
Conclusions: Inactivated SARS-CoV-2 vaccine may be a causal trigger leading to subacute thyroiditis. Clinicians should be aware of subacute thyroiditis as a possible thyroid-related side effect of an inactivated SARS-CoV-2 vaccine.
Keywords: BBIBP-CorV; coronavirus disease 2019; severe acute respiratory syndrome coronavirus 2; subacute thyroiditis; vaccine.
Copyright © 2022 Pi, Lin, Zheng, Wang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome.J Clin Endocrinol Metab. 2021 Aug 18;106(9):2600-2605. doi: 10.1210/clinem/dgab373. J Clin Endocrinol Metab. 2021. PMID: 34043800 Free PMC article.
-
Association of Homologous and Heterologous Vaccine Boosters With SARS-CoV-2 Infection in BBIBP-CorV Vaccinated Healthcare Personnel.Cureus. 2022 Jul 27;14(7):e27323. doi: 10.7759/cureus.27323. eCollection 2022 Jul. Cureus. 2022. PMID: 36043010 Free PMC article.
-
Altered T-cell receptor β repertoire in adults with SARS CoV-2 inactivated vaccine of BBIBP-CorV.Mol Immunol. 2023 Oct;162:54-63. doi: 10.1016/j.molimm.2023.08.005. Epub 2023 Aug 28. Mol Immunol. 2023. PMID: 37647774
-
Development of Graves' Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review.Front Public Health. 2021 Nov 23;9:778964. doi: 10.3389/fpubh.2021.778964. eCollection 2021. Front Public Health. 2021. PMID: 34888290 Free PMC article. Review.
-
Subacute Thyroiditis Following SARS-CoV-2 Vaccines: Six Cases Report and Review of the Literature.Horm Metab Res. 2022 Aug;54(8):556-561. doi: 10.1055/a-1804-9561. Epub 2022 Mar 22. Horm Metab Res. 2022. PMID: 35318621 Review.
Cited by
-
A case report of subacute thyroiditis after inactivated SARS-CoV-2 vaccine.SAGE Open Med Case Rep. 2022 Nov 28;10:2050313X221140243. doi: 10.1177/2050313X221140243. eCollection 2022. SAGE Open Med Case Rep. 2022. PMID: 36458024 Free PMC article.
-
Insights into SARS-CoV-2-associated subacute thyroiditis: from infection to vaccine.Virol J. 2023 Jun 21;20(1):132. doi: 10.1186/s12985-023-02103-1. Virol J. 2023. PMID: 37344878 Free PMC article. Review.
-
COVID-19 vaccination and thyroiditis.Best Pract Res Clin Endocrinol Metab. 2023 Jul;37(4):101759. doi: 10.1016/j.beem.2023.101759. Epub 2023 Mar 3. Best Pract Res Clin Endocrinol Metab. 2023. PMID: 36933997 Free PMC article. Review.
References
-
- Toft J, Larsen S, Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr J. (1998) 45:135. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

