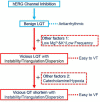
Vicious LQT induced by a combination of factors different from hERG inhibition
- PMID: 35935820
- PMCID: PMC9354841
- DOI: 10.3389/fphar.2022.930831
Vicious LQT induced by a combination of factors different from hERG inhibition
Abstract
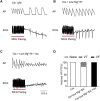
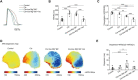
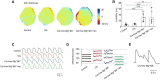
Clinically, drug-induced torsades de pointes (TdP) are rare events, whereas the reduction of the human ether-à-go-go-related gene (hERG) current is common. In this study, we aimed to explore the specific factors that contribute to the deterioration of hERG inhibition into malignant ventricular arrhythmias. Cisapride, a drug removed from the market because it caused long QT (LQT) syndrome and torsade de pointes (TdP), was used to induce hERG inhibition. The effects of cisapride on the hERG current were evaluated using a whole-cell patch clamp. Based on the dose-response curve of cisapride, models of its effects at different doses (10, 100, and 1,000 nM) on guinea pig heart in vitro were established. The effects of cisapride on electrocardiogram (ECG) signals and QT interval changes in the guinea pigs were then comprehensively evaluated by multi-channel electrical mapping and high-resolution fluorescence mapping, and changes in the action potential were simultaneously detected. Cisapride dose-dependently inhibited the hERG current with a half inhibitory concentration (IC50) of 32.63 ± 3.71 nM. The complete hERG suppression by a high dose of cisapride (1,000 nM) prolonged the action potential duration (APD), but not early after depolarizations (EADs) and TdP occurred. With 1 μM cisapride and lower Mg2+/K+, the APD exhibited triangulation, dispersion, and instability. VT was induced in two of 12 guinea pig hearts. Furthermore, the combined administration of isoproterenol was not therapeutic and increased susceptibility to ventricular fibrillation (VF) development. hERG inhibition alone led to QT and ERP prolongation and exerted an anti-arrhythmic effect. However, after the combination with low concentrations of magnesium and potassium, the prolonged action potential became unstable, triangular, and dispersed, and VT was easy to induce. The combination of catecholamines shortened the APD, but triangulation and dispersion still existed. At this time, VF was easily induced and sustained.
Keywords: QT prolongation; TDP; cisapride; dispersion; instability; optical mapping; triangulation.
Copyright © 2022 Xu, Yin, Li, Yao, Zhao, Wang, Wang, Dong, Zhang and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comparative pharmacology of guinea pig cardiac myocyte and cloned hERG (I(Kr)) channel.J Cardiovasc Electrophysiol. 2004 Nov;15(11):1302-9. doi: 10.1046/j.1540-8167.2004.04099.x. J Cardiovasc Electrophysiol. 2004. PMID: 15574182
-
A new biomarker--index of cardiac electrophysiological balance (iCEB)--plays an important role in drug-induced cardiac arrhythmias: beyond QT-prolongation and Torsades de Pointes (TdPs).J Pharmacol Toxicol Methods. 2013 Sep-Oct;68(2):250-259. doi: 10.1016/j.vascn.2013.01.003. Epub 2013 Jan 19. J Pharmacol Toxicol Methods. 2013. PMID: 23337247
-
Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines.Br J Pharmacol. 2008 Aug;154(7):1427-38. doi: 10.1038/bjp.2008.191. Epub 2008 May 19. Br J Pharmacol. 2008. PMID: 18493243 Free PMC article.
-
Refining detection of drug-induced proarrhythmia: QT interval and TRIaD.Heart Rhythm. 2005 Jul;2(7):758-72. doi: 10.1016/j.hrthm.2005.03.023. Heart Rhythm. 2005. PMID: 15992736 Review.
-
Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development.Cardiovasc Res. 2003 Apr 1;58(1):32-45. doi: 10.1016/s0008-6363(02)00846-5. Cardiovasc Res. 2003. PMID: 12667944 Review.
Cited by
-
Sequence-Dependent Repolarization Is Modulated by Endogenous Action Potential Duration Gradients Rather Than Electrical Coupling in Ventricular Myocardium.J Am Heart Assoc. 2025 Jan 7;14(1):e030433. doi: 10.1161/JAHA.123.030433. Epub 2024 Dec 24. J Am Heart Assoc. 2025. PMID: 39719415 Free PMC article.
References
LinkOut - more resources
Full Text Sources

