Dimethyl Itaconate Attenuates CFA-Induced Inflammatory Pain via the NLRP3/ IL-1β Signaling Pathway
- PMID: 35935847
- PMCID: PMC9353300
- DOI: 10.3389/fphar.2022.938979
Dimethyl Itaconate Attenuates CFA-Induced Inflammatory Pain via the NLRP3/ IL-1β Signaling Pathway
Abstract
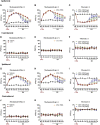
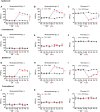
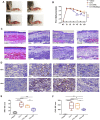
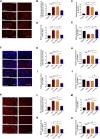
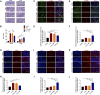
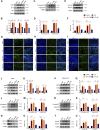
Itaconate plays a prominent role in anti-inflammatory effects and has gradually been ushered as a promising drug candidate for treating inflammatory diseases. However, its significance and underlying mechanism for inflammatory pain remain unexplored. In the current study, we investigated the effects and mechanisms of Dimethyl Itaconate (DI, a derivative of itaconate) on Complete Freund's adjuvant (CFA)-induced inflammatory pain in a rodent model. Here, we demonstrated that DI significantly reduced mechanical allodynia and thermal hyperalgesia. The DI-attenuated neuroinflammation was evident with the amelioration of infiltrative macrophages in peripheral sites of the hind paw and the dorsal root ganglion. Concurrently, DI hindered the central microglia activation in the spinal cord. Mechanistically, DI inhibited the expression of pro-inflammatory factors interleukin (IL)-1β and tumor necrosis factor alpha (TNF-α) and upregulated anti-inflammatory factor IL-10. The analgesic mechanism of DI was related to the downregulation of the nod-like receptor protein 3 (NLRP3) inflammasome complex and IL-1β secretion. This study suggested possible novel evidence for prospective itaconate utilization in the management of inflammatory pain.
Keywords: IL-1β; NLRP3 inflammasome complex; dimethyl itaconate; inflammatory pain; macrophages; microglia.
Copyright © 2022 Lin, Ren, Zhu, Dai, Gao, Xia, Cheng, Huang and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

